Chemistry:Pyraclostrobin
| |
| Names | |
|---|---|
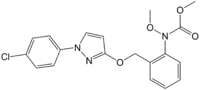
| IUPAC name
Methyl N-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]phenyl]-N-methoxycarbamate
| |
| Other names
Pyraclostrobine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H18ClN3O4 | |
| Molar mass | 387.82 g·mol−1 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H315, H331, H335, H410 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P311, P312, P321, P332+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyraclostrobin is a quinone outside inhibitor (QoI)-type[1][2][3][4] fungicide[5][6][7][8] used in agriculture.[8] Among the QoIs, it lies within the strobilurin chemical class.[9][8]
Use
Pyraclostrobin is used to protect Fragaria,[1][2][3] Rubus idaeus,[2] Vaccinium corymbosum,[2] Ribes rubrum,[2] Ribes uva-crispa,[2] blackberry (various Rubus spp.),[2] and Pistachio vera.[4]
Target pathogens
Pyraclostrobin is used against Botrytis cinerea[1][2][3] and Alternaria alternata.[4]
Resistance
Resistant populations have been identified in:
- Botrytis cinerea on Fragaria in the Carolinas, conferred by the G143A mutation in the partial cytochrome b (CYTB) gene.[1]
- Botrytis cinerea on Fragaria, Rubus idaeus, Vaccinium corymbosum, Ribes rubrum, Ribes uva-crispa, and blackberry (various Rubus spp.) in Northern Germany.[2]
- Botrytis cinerea on Fragaria in Florida.[3]
- Alternaria alternata on Pistachio vera in California .[4]
Geography of use
United States
Pyraclostrobin was widely used throughout the United States As of 2017[update], but especially in the Upper Midwest.[10][11]
Off-target toxicity
Although toxic, and recommended to be avoided by humans, pyraclostrobin is of temporary and low toxicity, that is to say it is merely an irritant[12] of eyes and skin.[8] It does cause some degree of reproductive and developmental failure in mammals[13] but does not absorb well through the skin.[8] It is likely to bioaccumulate in aquatic organisms.[8]
Residues in diet
Pyraclostrobin does not accumulate in foods to a significant degree.[9]
Biodegradability
Pyraclostrobin is described by one source as not very biodegradable,[9] and by another as possibly significantly biodegradable.[8]
References
- ↑ 1.0 1.1 1.2 1.3 Fernández-Ortuño, Dolores; Chen, Fengping; Schnabel, Guido (2012). "Resistance to Pyraclostrobin and Boscalid in Botrytis cinerea Isolates from Strawberry Fields in the Carolinas". Plant Disease (American Phytopathological Society) 96 (8): 1198–1203. doi:10.1094/pdis-12-11-1049-re. ISSN 0191-2917. PMID 30727059.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Weber, Roland W. S. (2011). "Resistance of Botrytis cinerea to Multiple Fungicides in Northern German Small-Fruit Production". Plant Disease (American Phytopathological Society) 95 (10): 1263–1269. doi:10.1094/pdis-03-11-0209. ISSN 0191-2917. PMID 30731691.
- ↑ 3.0 3.1 3.2 3.3 Amiri, A.; Heath, S. M.; Peres, N. A. (2013). "Phenotypic Characterization of Multifungicide Resistance in Botrytis cinerea Isolates from Strawberry Fields in Florida". Plant Disease (American Phytopathological Society) 97 (3): 393–401. doi:10.1094/pdis-08-12-0748-re. ISSN 0191-2917. PMID 30722364.
- ↑ 4.0 4.1 4.2 4.3 Avenot, H.; Morgan, D. P.; Michailides, T. J. (2007-09-16). "Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin + boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California". Plant Pathology (British Society for Plant Pathology/Wiley-Blackwell) 57 (1): 135–140. doi:10.1111/j.1365-3059.2007.01701.x. ISSN 0032-0862.
- ↑ "Pyraclostrobin". United States Environmental Protection Agency. http://sor.epa.gov/sor_internet/registry/substreg/searchandretrieve/advancedsearch/externalSearch.do?p_type=CASNO&p_value=175013-18-0.
- ↑ Chambers, Michael. "175013-18-0 - HZRSNVGNWUDEFX-UHFFFAOYSA-N - Pyraclostrobin [ISO:BSI - Similar structures search, synonyms, formulas, resource links, and other chemical information."]. National Library of Medicine, US NIH. https://chem.nlm.nih.gov/chemidplus/rn/175013-18-0.
- ↑ "Preferred Substance Name: PYRACLOSTROBIN UNII: DJW8M9OX1H". US Food and Drug Administration. http://fdasis.nlm.nih.gov/srs/unii/DJW8M9OX1H.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 "Pyraclostrobin". NCBI, NLM, US NIH. http://pubchem.ncbi.nlm.nih.gov/compound/6422843.
- ↑ 9.0 9.1 9.2 Declercq, Bernard. "Pyraclostrobin (210)". Épinay-sur-Orge, France: UN FAO (Food and Agriculture Organization of the United Nations. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation04/Pyraclostrobinaf.pdf.
- ↑ "2017 Pesticide Use Map - Pyraclostrobin". United States Geological Survey (USGS). http://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2017&map=PYRACLOSTROBIN&hilo=L.
- ↑ "2017 Pesticide Use Map - Pyraclostrobin". United States Geological Survey (USGS). http://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2017&map=PYRACLOSTROBIN&hilo=H.
- ↑ "Acute Pesticide Poisoning Associated with Pyraclostrobin Fungicide --- Iowa, 2007". 2008-01-04. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5651a3.htm.
- ↑ Health Risk Assessment Unit (June 2016). "Toxicological Summary for: Pyraclostrobin". Environmental Health Division, Minnesota Department of Health. http://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/pyrac.pdf.
Further reading
- "CID 5327095". NCBI, NLM, US NIH. http://pubchem.ncbi.nlm.nih.gov/compound/5327095.
- "Pyraclostrobine". National Institute of Standards and Technology. http://webbook.nist.gov/cgi/inchi/InChI%3D1S/C19H18ClN3O4/c1-25-19(24)23(26-2)17-6-4-3-5-14(17)13-27-18-11-12-22(21-18)16-9-7-15(20)8-10-16/h3-12H%2C13H2%2C1-2H3.
 |
