Chemistry:Ralimetinib
From HandWiki
Short description: Chemical compound
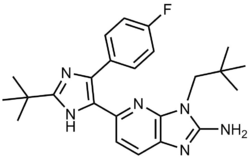 Ralimetinib structural formula | |
| Clinical data | |
|---|---|
| Other names | LY2228820 |
| Routes of administration | PO |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C24H29FN6 |
| Molar mass | 420.536 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ralimetinib (LY2228820) is a small molecule experimental cancer drug in development by Eli Lilly. Although originally thought to be a p38 mitogen-activated protein kinase (MAPK) inhibitor,[1] it has since been reported that it acts instead as an epidermal growth factor receptor (EGFR) inhibitor.[2]
A phase II trial for treatment of ovarian cancer has completed.[3][4]
References
- ↑ "A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer". Clinical Cancer Research 22 (5): 1095–1102. March 2016. doi:10.1158/1078-0432.CCR-15-1718. PMID 26581242.
- ↑ "Inhibition of a lower potency target drives the anticancer activity of a clinical p38 inhibitor". Cell Chemical Biology 30 (10): 1211–1222.e5. October 2023. doi:10.1016/j.chembiol.2023.09.013. PMID 37827156.
- ↑ Clinical trial number NCT01663857 for "A Study LY2228820 for Recurrent Ovarian Cancer" at ClinicalTrials.gov
- ↑ "A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer". Gynecologic Oncology 156 (1): 23–31. January 2020. doi:10.1016/j.ygyno.2019.11.006. PMID 31791552.
 |

