Biology:Epidermal growth factor receptor
 Generic protein structure example |
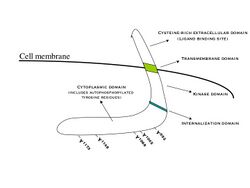
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a transmembrane protein that is a receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands.[1]
The epidermal growth factor receptor is a member of the ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). In many cancer types, mutations affecting EGFR expression or activity could result in cancer.[2]
Epidermal growth factor and its receptor was discovered by Stanley Cohen of Vanderbilt University. Cohen shared the 1986 Nobel Prize in Medicine with Rita Levi-Montalcini for their discovery of growth factors.
Deficient signaling of the EGFR and other receptor tyrosine kinases in humans is associated with diseases such as Alzheimer's, while over-expression is associated with the development of a wide variety of tumors. Interruption of EGFR signalling, either by blocking EGFR binding sites on the extracellular domain of the receptor or by inhibiting intracellular tyrosine kinase activity, can prevent the growth of EGFR-expressing tumours and improve the patient's condition[citation needed].
Function
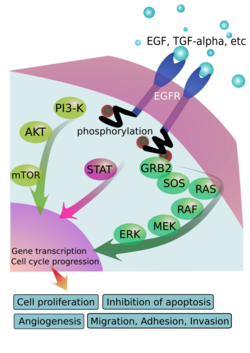
Epidermal growth factor receptor (EGFR) is a transmembrane protein that is activated by binding of its specific ligands, including epidermal growth factor and transforming growth factor alpha (TGF-α).[3] ErbB2 has no known direct activating ligand, and may be in an activated state constitutively or become active upon heterodimerization with other family members such as EGFR. Upon activation by its growth factor ligands, EGFR undergoes a transition from an inactive monomeric form to an active homodimer.[4] – although there is some evidence that preformed inactive dimers may also exist before ligand binding.[5] In addition to forming homodimers after ligand binding, EGFR may pair with another member of the ErbB receptor family, such as ErbB2/Her2/neu, to create an activated heterodimer. There is also evidence to suggest that clusters of activated EGFRs form, although it remains unclear whether this clustering is important for activation itself or occurs subsequent to activation of individual dimers.[6]
EGFR dimerization stimulates its intrinsic intracellular protein-tyrosine kinase activity. As a result, autophosphorylation of several tyrosine (Y) residues in the C-terminal domain of EGFR occurs. These include Y992, Y1045, Y1068, Y1148 and Y1173, as shown in the adjacent diagram.[7] This autophosphorylation elicits downstream activation and signaling by several other proteins that associate with the phosphorylated tyrosines through their own phosphotyrosine-binding SH2 domains. These downstream signaling proteins initiate several signal transduction cascades, principally the MAPK, Akt and JNK pathways, leading to DNA synthesis and cell proliferation.[8] Such proteins modulate phenotypes such as cell migration, adhesion, and proliferation. Activation of the receptor is important for the innate immune response in human skin. Additionally, the kinase domain of the EGFR can cross-phosphorylate the tyrosine residues of other receptors with which it is aggregated and thereby activate itself.
Biological roles
The EGFR is essential for ductal development of the mammary glands,[9][10][11] and agonists of the EGFR such as amphiregulin, TGF-α, and heregulin induce both ductal and lobuloalveolar development even in the absence of estrogen and progesterone.[12][13]
Role in human disease
Cancer
Mutations that lead to EGFR overexpression (known as upregulation or amplification) have been associated with a number of cancers, including adenocarcinoma of the lung (40% of cases), anal cancers,[14] glioblastoma (50%) and epithelian tumors of the head and neck (80–100%).[15] These somatic mutations involving EGFR lead to its constant activation, which produces uncontrolled cell division.[16] In glioblastoma a specific mutation of EGFR, called EGFRvIII, is often observed.[17] Mutations, amplifications or misregulations of EGFR or family members are implicated in about 30% of all epithelial cancers.[18]
Inflammatory disease
Aberrant EGFR signaling has been implicated in psoriasis, eczema and atherosclerosis.[19][20] However, its exact roles in these conditions are ill-defined.
Monogenic disease
A single child displaying multi-organ epithelial inflammation was found to have a homozygous loss of function mutation in the EGFR gene. The pathogenicity of the EGFR mutation was supported by in vitro experiments and functional analysis of a skin biopsy. His severe phenotype reflects many previous research findings into EGFR function. His clinical features included a papulopustular rash, dry skin, chronic diarrhoea, abnormalities of hair growth, breathing difficulties and electrolyte imbalances.[21]
Wound healing and fibrosis
EGFR has been shown to play a critical role in TGF-beta1 dependent fibroblast to myofibroblast differentiation.[22][23] Aberrant persistence of myofibroblasts within tissues can lead to progressive tissue fibrosis, impairing tissue or organ function (e.g. skin hypertrophic or keloid scars, liver cirrhosis, myocardial fibrosis, chronic kidney disease).
Medical applications
Drug target
The identification of EGFR as an oncogene has led to the development of anticancer therapeutics directed against EGFR (called "EGFR inhibitors", EGFRi), including gefitinib,[24] erlotinib,[25] afatinib, brigatinib and icotinib[26][27] for lung cancer, and cetuximab for colon cancer. More recently AstraZeneca has developed Osimertinib, a third generation tyrosine kinase inhibitor.[28][27]
Many therapeutic approaches are aimed at the EGFR. Cetuximab and panitumumab are examples of monoclonal antibody inhibitors. However the former is of the IgG1 type, the latter of the IgG2 type; consequences on antibody-dependent cellular cytotoxicity can be quite different.[29] Other monoclonals in clinical development are zalutumumab, nimotuzumab, and matuzumab. The monoclonal antibodies block the extracellular ligand binding domain. With the binding site blocked, signal molecules can no longer attach there and activate the tyrosine kinase.
Another method is using small molecules to inhibit the EGFR tyrosine kinase, which is on the cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to activate itself, which is a prerequisite for binding of downstream adaptor proteins. Ostensibly by halting the signaling cascade in cells that rely on this pathway for growth, tumor proliferation and migration is diminished. Gefitinib, erlotinib, brigatinib and lapatinib (mixed EGFR and ERBB2 inhibitor) are examples of small molecule kinase inhibitors.
CimaVax-EGF, an active vaccine targeting EGF as the major ligand of EGF, uses a different approach, raising antibodies against EGF itself, thereby denying EGFR-dependent cancers of a proliferative stimulus;[30] it is in use as a cancer therapy against non-small-cell lung carcinoma (the most common form of lung cancer) in Cuba, and is undergoing further trials for possible licensing in Japan, Europe, and the United States.[31]
There are several quantitative methods available that use protein phosphorylation detection to identify EGFR family inhibitors.[32]
New drugs such as osimertinib, gefitinib, erlotinib and brigatinib directly target the EGFR. Patients have been divided into EGFR-positive and EGFR-negative, based upon whether a tissue test shows a mutation. EGFR-positive patients have shown a 60% response rate, which exceeds the response rate for conventional chemotherapy.[33]
However, many patients develop resistance. Two primary sources of resistance are the T790M mutation and MET oncogene.[33] However, as of 2010 there was no consensus of an accepted approach to combat resistance nor FDA approval of a specific combination. Clinical trial phase II results reported for brigatinib targeting the T790M mutation, and brigatinib received Breakthrough Therapy designation status by FDA in Feb. 2015.
The most common adverse effect of EGFR inhibitors, found in more than 90% of patients, is a papulopustular rash that spreads across the face and torso; the rash's presence is correlated with the drug's antitumor effect.[34] In 10% to 15% of patients the effects can be serious and require treatment.[35][36]
Some tests are aiming at predicting benefit from EGFR treatment, as Veristrat.[37]
Laboratory research using genetically engineered stem cells to target EGFR in mice was reported in 2014 to show promise.[38] EGFR is a well-established target for monoclonal antibodies and specific tyrosine kinase inhibitors.[39]
Target for imaging agents
Imaging agents have been developed which identify EGFR-dependent cancers using labeled EGF.[40] The feasibility of in vivo imaging of EGFR expression has been demonstrated in several studies.[41][42]
It has been proposed that certain computed tomography findings such as ground-glass opacities, air bronchogram, spiculated margins, vascular convergence, and pleural retraction can predict the presence of EGFR mutation in patients with non-small cell lung cancer.[43]
Interactions
Epidermal growth factor receptor has been shown to interact with:
- AR,[44][45]
- ARF4,[46]
- CAV1,[47]
- CAV3,[47]
- CBL,[48][49][50][51][52]
- CBLB,[49][53]
- CBLC,[54][55]
- CD44,[22]
- CDC25A,[56]
- CRK,[53][57]
- CTNNB1,[58][59][60]
- DCN,[61][62]
- EGF,[63][64]
- GRB14,[65]
- Grb2,[53][63][65][66][67][68][69][70][71][72]
- JAK2,[73]
- MUC1,[74][75]
- NCK1,[66][76][77]
- NCK2[66][78][79]
- PKC alpha,[80]
- PLCG1,[48][81]
- PLSCR1,[82]
- PTPN1,[83][84]
- PTPN11,[53][85]
- PTPN6,[85][86]
- PTPRK,[87]
- SH2D3A,[88]
- SH3KBP1,[89][90]
- SHC1,[53][91]
- SOS1,[71][92][93]
- Src,[73][94][95]
- STAT1,[73][96]
- STAT3,[73][97]
- STAT5A,[53][73]
- UBC,[50][51][98] and
- WAS,[99]
- PAR2.[100]
In fruitflies, the epidermal growth factor receptor interacts with Spitz.[101]
References
- ↑ "Review of epidermal growth factor receptor biology". International Journal of Radiation Oncology, Biology, Physics 59 (2 Suppl): 21–6. 2004. doi:10.1016/j.ijrobp.2003.11.041. PMID 15142631.
- ↑ "ErbB receptors: from oncogenes to targeted cancer treatment". The Journal of Clinical Investigation 117 (8): 2051–8. August 2007. doi:10.1172/JCI32278. PMID 17671639.
- ↑ note, a full list of the ligands able to activate EGFR and other members of the ErbB family is given in the ErbB article)
- ↑ "Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor". Biochemistry 26 (5): 1443–51. March 1987. doi:10.1021/bi00379a035. PMID 3494473.
- ↑ "Mechanisms of activation of receptor tyrosine kinases: monomers or dimers". Cells 3 (2): 304–30. April 2014. doi:10.3390/cells3020304. PMID 24758840.
- ↑ "Nanoscale imaging of epidermal growth factor receptor clustering: effects of inhibitors" (in English). The Journal of Biological Chemistry 285 (5): 3145–3156. January 2010. doi:10.1074/jbc.M109.073338. PMID 19959837.
- ↑ "Autophosphorylation sites on the epidermal growth factor receptor". Nature 311 (5985): 483–5. 1984. doi:10.1038/311483a0. PMID 6090945. Bibcode: 1984Natur.311..483D.
- ↑ "A comprehensive pathway map of epidermal growth factor receptor signaling". Molecular Systems Biology 1 (1): E1–E17. 2005. doi:10.1038/msb4100014. PMID 16729045.
- ↑ "Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis". Cell Growth & Differentiation 9 (9): 777–85. September 1998. PMID 9751121.
- ↑ "Amphiregulin: role in mammary gland development and breast cancer". Journal of Mammary Gland Biology and Neoplasia 13 (2): 159–69. June 2008. doi:10.1007/s10911-008-9075-7. PMID 18398673.
- ↑ "The ADAM17-amphiregulin-EGFR axis in mammary development and cancer". Journal of Mammary Gland Biology and Neoplasia 13 (2): 181–94. June 2008. doi:10.1007/s10911-008-9084-6. PMID 18470483.
- ↑ "Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse". Breast Cancer Research and Treatment 79 (2): 161–73. May 2003. doi:10.1023/a:1023938510508. PMID 12825851.
- ↑ "Induction of ductal morphogenesis and lobular hyperplasia by amphiregulin in the mouse mammary gland". Cell Growth & Differentiation 7 (12): 1769–81. December 1996. PMID 8959346.
- ↑ "Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus". Human Pathology 40 (11): 1517–27. November 2009. doi:10.1016/j.humpath.2009.05.010. PMID 19716155.
- ↑ Robbins basic pathology. Philadelphia: Elsevier/Saunders. 2013. p. 179. ISBN 9781437717815.
- ↑ "Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib". The New England Journal of Medicine 350 (21): 2129–39. May 2004. doi:10.1056/NEJMoa040938. PMID 15118073. http://repository.cshl.edu/22429/1/EGFR%20Mutations.pdf.
- ↑ "EGF mutant receptor vIII as a molecular target in cancer therapy". Endocrine-Related Cancer 8 (2): 83–96. June 2001. doi:10.1677/erc.0.0080083. PMID 11397666.
- ↑ "Knockdown of EGFR inhibits growth and invasion of gastric cancer cells". Cancer Gene Therapy 21 (11): 491–7. November 2014. doi:10.1038/cgt.2014.55. PMID 25394504.
- ↑ "The EGF receptor – an essential regulator of multiple epidermal functions". European Journal of Dermatology 10 (7): 505–10. 2000. PMID 11056418.
- ↑ "The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis". Atherosclerosis 186 (1): 38–53. May 2006. doi:10.1016/j.atherosclerosis.2005.06.038. PMID 16076471.
- ↑ "Epithelial inflammation resulting from an inherited loss-of-function mutation in EGFR". The Journal of Investigative Dermatology 134 (10): 2570–8. October 2014. doi:10.1038/jid.2014.164. PMID 24691054.
- ↑ 22.0 22.1 "Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts". The Journal of Biological Chemistry 288 (21): 14824–38. May 2013. doi:10.1074/jbc.M113.451336. PMID 23589287.
- ↑ "MicroRNA-7 inhibition rescues age-associated loss of epidermal growth factor receptor and hyaluronan-dependent differentiation in fibroblasts". Aging Cell 13 (2): 235–44. April 2014. doi:10.1111/acel.12167. PMID 24134702.
- ↑ "EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy". Science 304 (5676): 1497–1500. June 2004. doi:10.1126/science.1099314. PMID 15118125. Bibcode: 2004Sci...304.1497P.
- ↑ "Randomised controlled trial of first-line tyrosine-kinase inhibitor (TKI) versus intercalated TKI with chemotherapy for EGFR-mutated nonsmall cell lung cancer". ERJ Open Research 8 (4): 00239–2022. October 2022. doi:10.1183/23120541.00239-2022. PMID 36267895.
- ↑ "Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations". PLOS ONE 9 (2): e85245. 12 February 2014. doi:10.1371/journal.pone.0085245. PMID 24533047. Bibcode: 2014PLoSO...985245L.
- ↑ 27.0 27.1 "Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: a retrospective, nationwide registry study". The Lancet Regional Health. Europe 27: 100592. April 2023. doi:10.1016/j.lanepe.2023.100592. PMID 36817181.
- ↑ "Osimertinib: First Global Approval". Drugs 76 (2): 263–273. February 2016. doi:10.1007/s40265-015-0533-4. PMID 26729184.
- ↑ "Pharmacogenetics and pharmacogenomics in oncology therapeutic antibody development". BioTechniques 39 (4): 565–8. October 2005. doi:10.2144/000112043. PMID 16235569.
- ↑ "Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy". MEDICC Review 12 (1): 17–23. Winter 2010. doi:10.37757/MR2010.V12.N1.4. PMID 20387330.
- ↑ "Cuba Has a Lung Cancer Vaccine—And America Wants It". Wired. 11 May 2015. https://www.wired.com/2015/05/cimavax-roswell-park-cancer-institute/. Retrieved 13 May 2015.
- ↑ "Quantitative methods for the analysis of protein phosphorylation in drug development". Expert Review of Proteomics 1 (3): 327–41. October 2004. doi:10.1586/14789450.1.3.327. PMID 15966829.
- ↑ 33.0 33.1 "Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials". Clinical Cancer Research 15 (16): 5267–73. August 2009. doi:10.1158/1078-0432.CCR-09-0888. PMID 19671843.
- ↑ "Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis". PLOS ONE 8 (1): e55128. 2013. doi:10.1371/journal.pone.0055128. PMID 23383079. Bibcode: 2013PLoSO...855128L.
- ↑ "Management of EGFR-inhibitor associated rash: a retrospective study in 49 patients". European Journal of Medical Research 17 (1): 4. 2012. doi:10.1186/2047-783X-17-4. PMID 22472354.
- ↑ "Mechanisms of cutaneous toxicities to EGFR inhibitors". Nature Reviews. Cancer 6 (10): 803–12. October 2006. doi:10.1038/nrc1970. PMID 16990857.
- ↑ "VeriStrat: a prognostic and/or predictive biomarker for advanced lung cancer patients?". Expert Review of Respiratory Medicine 8 (1): 1–4. February 2014. doi:10.1586/17476348.2014.861744. PMID 24308656.
- ↑ "Engineering toxin-resistant therapeutic stem cells to treat brain tumors". Stem Cells 33 (2): 589–600. February 2015. doi:10.1002/stem.1874. PMID 25346520.
- ↑ "The ErbB/HER family of protein-tyrosine kinases and cancer". Pharmacological Research 79: 34–74. January 2014. doi:10.1016/j.phrs.2013.11.002. PMID 24269963.
- ↑ "Development of a sensitive, stable and EGFR-specific molecular imaging agent for surface enhanced Raman spectroscopy". Journal of Raman Spectroscopy 46 (5): 434–446. May 2015. doi:10.1002/jrs.4678. Bibcode: 2015JRSp...46..434L.
- ↑ "Aggregation of nanoparticles in endosomes and lysosomes produces surface-enhanced Raman spectroscopy". Journal of Nanophotonics 9 (1): 093094–1–14. 23 January 2015. doi:10.1117/1.JNP.9.093094. Bibcode: 2015JNano...9.3094L.
- ↑ "Feasibility of imaging of epidermal growth factor receptor expression with ZEGFR:2377 affibody molecule labeled with 99mTc using a peptide-based cysteine-containing chelator". International Journal of Oncology 49 (6): 2285–2293. December 2016. doi:10.3892/ijo.2016.3721. PMID 27748899.
- ↑ Herrera Ortiz AF, Cadavid Camacho T, Vásquez Perdomo A, Castillo Herazo V, Arambula Neira J, Yepes Bustamante M, Cadavid Camacho E. Clinical and CT patterns to predict EGFR mutation in patients with non-small cell lung cancer: A systematic literature review and meta-analysis. European Journal of Radiology Open.2022;9:100400. https://doi.org/10.1016/j.ejro.2022.100400
- ↑ "EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR)". International Journal of Cancer 112 (1): 78–86. October 2004. doi:10.1002/ijc.20362. PMID 15305378.
- ↑ "The androgen receptor associates with the epidermal growth factor receptor in androgen-sensitive prostate cancer cells". Steroids 69 (8–9): 549–52. August 2004. doi:10.1016/j.steroids.2004.05.011. PMID 15288768.
- ↑ "ADP-ribosylation factor 4 small GTPase mediates epidermal growth factor receptor-dependent phospholipase D2 activation". The Journal of Biological Chemistry 278 (4): 2661–8. January 2003. doi:10.1074/jbc.M205819200. PMID 12446727.
- ↑ 47.0 47.1 "Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities". The Journal of Biological Chemistry 272 (48): 30429–38. November 1997. doi:10.1074/jbc.272.48.30429. PMID 9374534.
- ↑ 48.0 48.1 "EGF-dependent association of phospholipase C-gamma1 with c-Cbl". Experimental Cell Research 277 (1): 86–94. July 2002. doi:10.1006/excr.2002.5545. PMID 12061819.
- ↑ 49.0 49.1 "cbl-b inhibits epidermal growth factor receptor signaling". Oncogene 18 (10): 1855–66. March 1999. doi:10.1038/sj.onc.1202499. PMID 10086340.
- ↑ 50.0 50.1 "A tale of two Cbls: interplay of c-Cbl and Cbl-b in epidermal growth factor receptor downregulation". Molecular and Cellular Biology 28 (9): 3020–37. May 2008. doi:10.1128/MCB.01809-07. PMID 18316398.
- ↑ 51.0 51.1 "Ubc4/5 and c-Cbl continue to ubiquitinate EGF receptor after internalization to facilitate polyubiquitination and degradation". Molecular Biology of the Cell 19 (8): 3454–62. August 2008. doi:10.1091/mbc.E07-10-0988. PMID 18508924.
- ↑ "Structural basis for a novel intrapeptidyl H-bond and reverse binding of c-Cbl-TKB domain substrates". The EMBO Journal 27 (5): 804–16. March 2008. doi:10.1038/emboj.2008.18. PMID 18273061.
- ↑ 53.0 53.1 53.2 53.3 53.4 53.5 "Phosphotyrosine interactome of the ErbB-receptor kinase family". Molecular Systems Biology 1 (1): E1–E13. 2005. doi:10.1038/msb4100012. PMID 16729043.
- ↑ "Molecular cloning and characterization of a novel cbl-family gene, cbl-c". Gene 239 (1): 145–54. October 1999. doi:10.1016/S0378-1119(99)00356-X. PMID 10571044.
- ↑ "cbl-3: a new mammalian cbl family protein". Oncogene 18 (22): 3365–75. June 1999. doi:10.1038/sj.onc.1202753. PMID 10362357.
- ↑ "Identification of epidermal growth factor receptor as a target of Cdc25A protein phosphatase". The Journal of Biological Chemistry 277 (22): 19470–5. May 2002. doi:10.1074/jbc.M201097200. PMID 11912208.
- ↑ "Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor". The Journal of Biological Chemistry 273 (27): 17186–91. July 1998. doi:10.1074/jbc.273.27.17186. PMID 9642287.
- ↑ "The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton". The Journal of Biological Chemistry 273 (15): 9078–84. April 1998. doi:10.1074/jbc.273.15.9078. PMID 9535896.
- ↑ "ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas". The Journal of Biological Chemistry 277 (25): 22692–8. June 2002. doi:10.1074/jbc.M201975200. PMID 11950845.
- ↑ "Induction of tyrosine phosphorylation and association of beta-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence". Oncogene 15 (1): 71–8. July 1997. doi:10.1038/sj.onc.1201160. PMID 9233779.
- ↑ "Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope". The Journal of Biological Chemistry 277 (38): 35671–81. September 2002. doi:10.1074/jbc.M205317200. PMID 12105206.
- ↑ "Decorin is a biological ligand for the epidermal growth factor receptor". The Journal of Biological Chemistry 274 (8): 4489–92. February 1999. doi:10.1074/jbc.274.8.4489. PMID 9988678.
- ↑ 63.0 63.1 "A differential requirement for the COOH-terminal region of the epidermal growth factor (EGF) receptor in amphiregulin and EGF mitogenic signaling". The Journal of Biological Chemistry 274 (13): 8900–9. March 1999. doi:10.1074/jbc.274.13.8900. PMID 10085134.
- ↑ "Role of the N-terminus of epidermal growth factor in ErbB-2/ErbB-3 binding studied by phage display". Biochemistry 41 (27): 8732–41. July 2002. doi:10.1021/bi025878c. PMID 12093292.
- ↑ 65.0 65.1 "Cloning and characterization of GRB14, a novel member of the GRB7 gene family". The Journal of Biological Chemistry 271 (21): 12502–10. May 1996. doi:10.1074/jbc.271.21.12502. PMID 8647858.
- ↑ 66.0 66.1 66.2 "Identification of Grb4/Nckbeta, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck". The Journal of Biological Chemistry 274 (9): 5542–9. February 1999. doi:10.1074/jbc.274.9.5542. PMID 10026169.
- ↑ "A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling". Nature Biotechnology 21 (3): 315–8. March 2003. doi:10.1038/nbt790. PMID 12577067.
- ↑ "UCS15A, a novel small molecule, SH3 domain-mediated protein-protein interaction blocking drug". Oncogene 21 (13): 2037–50. March 2002. doi:10.1038/sj.onc.1205271. PMID 11960376.
- ↑ "Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells". The Journal of Biological Chemistry 269 (49): 31310–4. December 1994. doi:10.1016/S0021-9258(18)47424-8. PMID 7527043.
- ↑ "The RIalpha subunit of protein kinase A (PKA) binds to Grb2 and allows PKA interaction with the activated EGF-receptor". Oncogene 14 (8): 923–8. February 1997. doi:10.1038/sj.onc.1200906. PMID 9050991.
- ↑ 71.0 71.1 "A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells". The Journal of Biological Chemistry 269 (12): 9019–23. March 1994. doi:10.1016/S0021-9258(17)37070-9. PMID 7510700.
- ↑ "The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling". Cell 70 (3): 431–42. August 1992. doi:10.1016/0092-8674(92)90167-B. PMID 1322798.
- ↑ 73.0 73.1 73.2 73.3 73.4 "ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases". The Journal of Biological Chemistry 274 (24): 17209–18. June 1999. doi:10.1074/jbc.274.24.17209. PMID 10358079.
- ↑ "Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland". The Journal of Biological Chemistry 276 (16): 13057–64. April 2001. doi:10.1074/jbc.M011248200. PMID 11278868.
- ↑ "The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin". The Journal of Biological Chemistry 276 (38): 35239–42. September 2001. doi:10.1074/jbc.C100359200. PMID 11483589.
- ↑ "Induced direct binding of the adapter protein Nck to the GTPase-activating protein-associated protein p62 by epidermal growth factor". Oncogene 15 (15): 1823–32. October 1997. doi:10.1038/sj.onc.1201351. PMID 9362449.
- ↑ "The SH2 and SH3 domain-containing Nck protein is oncogenic and a common target for phosphorylation by different surface receptors". Molecular and Cellular Biology 12 (12): 5824–33. December 1992. doi:10.1128/MCB.12.12.5824. PMID 1333047.
- ↑ "Identification of Nck family genes, chromosomal localization, expression, and signaling specificity". The Journal of Biological Chemistry 273 (39): 25171–8. September 1998. doi:10.1074/jbc.273.39.25171. PMID 9737977.
- ↑ "Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways". Molecular Biology of the Cell 9 (12): 3367–82. December 1998. doi:10.1091/mbc.9.12.3367. PMID 9843575.
- ↑ "Protein kinase Calpha negatively regulates cell spreading and motility in MDA-MB-231 human breast cancer cells downstream of epidermal growth factor receptor". Biochemical and Biophysical Research Communications 307 (4): 839–46. August 2003. doi:10.1016/S0006-291X(03)01273-7. PMID 12878187.
- ↑ "Cytoskeletal association of epidermal growth factor receptor and associated signaling proteins is regulated by cell density in IEC-6 intestinal cells". Journal of Cellular Physiology 172 (1): 126–36. July 1997. doi:10.1002/(SICI)1097-4652(199707)172:1<126::AID-JCP14>3.0.CO;2-A. PMID 9207933.
- ↑ "Plasma membrane phospholipid scramblase 1 is enriched in lipid rafts and interacts with the epidermal growth factor receptor". Biochemistry 41 (20): 6338–45. May 2002. doi:10.1021/bi025610l. PMID 12009895.
- ↑ "Structural basis of plasticity in protein tyrosine phosphatase 1B substrate recognition". Biochemistry 39 (28): 8171–9. July 2000. doi:10.1021/bi000319w. PMID 10889023.
- ↑ "Determinants of substrate recognition in the protein-tyrosine phosphatase, PTP1". The Journal of Biological Chemistry 271 (10): 5386–92. March 1996. doi:10.1074/jbc.271.10.5386. PMID 8621392.
- ↑ 85.0 85.1 "Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C". The Journal of Biological Chemistry 270 (36): 21277–84. September 1995. doi:10.1074/jbc.270.36.21277. PMID 7673163.
- ↑ "Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling". The Journal of Biological Chemistry 273 (38): 24839–46. September 1998. doi:10.1074/jbc.273.38.24839. PMID 9733788.
- ↑ "Transforming growth factor {beta} (TGF-{beta})-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-{beta} function". Molecular and Cellular Biology 25 (11): 4703–15. June 2005. doi:10.1128/MCB.25.11.4703-4715.2005. PMID 15899872.
- ↑ "NSP1 defines a novel family of adaptor proteins linking integrin and tyrosine kinase receptors to the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway". The Journal of Biological Chemistry 274 (15): 10047–52. April 1999. doi:10.1074/jbc.274.15.10047. PMID 10187783.
- ↑ "Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors". Nature 416 (6877): 183–7. March 2002. doi:10.1038/416183a. PMID 11894095. Bibcode: 2002Natur.416..183S.
- ↑ "CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases". The Journal of Biological Chemistry 277 (42): 39666–72. October 2002. doi:10.1074/jbc.M205535200. PMID 12177062.
- ↑ "Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation in intact cells". Molecular Endocrinology 12 (4): 536–43. April 1998. doi:10.1210/mend.12.4.0094. PMID 9544989.
- ↑ "The Sos1 and Sos2 Ras-specific exchange factors: differences in placental expression and signaling properties". The EMBO Journal 19 (4): 642–54. February 2000. doi:10.1093/emboj/19.4.642. PMID 10675333.
- ↑ "N terminus of Sos1 Ras exchange factor: critical roles for the Dbl and pleckstrin homology domains". Molecular and Cellular Biology 18 (2): 771–8. February 1998. doi:10.1128/mcb.18.2.771. PMID 9447973.
- ↑ "Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular Ca2+, PYK-2, and p60(src)". The Journal of Biological Chemistry 275 (17): 12619–25. April 2000. doi:10.1074/jbc.275.17.12619. PMID 10777553.
- ↑ "Adaptor protein Shc undergoes translocation and mediates up-regulation of the tyrosine kinase c-Src in EGF-stimulated A431 cells". Genes to Cells 5 (9): 749–64. September 2000. doi:10.1046/j.1365-2443.2000.00358.x. PMID 10971656.
- ↑ "Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation". The Journal of Biological Chemistry 277 (34): 30716–23. August 2002. doi:10.1074/jbc.M202823200. PMID 12070153.
- ↑ "Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells". Molecular and Cellular Biology 24 (21): 9390–400. November 2004. doi:10.1128/MCB.24.21.9390-9400.2004. PMID 15485908.
- ↑ "Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis". Cancer Research 68 (14): 5669–77. July 2008. doi:10.1158/0008-5472.CAN-07-6364. PMID 18632619.
- ↑ "Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells". Molecular Biology of the Cell 8 (9): 1709–21. September 1997. doi:10.1091/mbc.8.9.1709. PMID 9307968.
- ↑ "PAR2 induces ovarian cancer cell motility by merging three signalling pathways to transactivate EGFR". British Journal of Pharmacology (n/a) ((n/a)): 913–932. November 2020. doi:10.1111/bph.15332. ISSN 0007-1188. PMID 33226635.
- ↑ "Signaling by the Drosophila epidermal growth factor receptor pathway during development". Experimental Cell Research 284 (1): 140–9. March 2003. doi:10.1016/S0014-4827(02)00094-0. PMID 12648473.
Further reading
- "Receptors for epidermal growth factor and other polypeptide mitogens". Annual Review of Biochemistry 56 (1): 881–914. 1987. doi:10.1146/annurev.bi.56.070187.004313. PMID 3039909.
- "The epidermal growth factor". Cell Biology International 19 (5): 413–30. May 1995. doi:10.1006/cbir.1995.1086. PMID 7640657.
- "The EGF receptor: a nexus for trafficking and signaling". BioEssays 22 (8): 697–707. August 2000. doi:10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. PMID 10918300.
- "Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer". The Journal of Steroid Biochemistry and Molecular Biology 80 (2): 231–8. February 2002. doi:10.1016/S0960-0760(01)00190-X. PMID 11897506.
- "Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor". IUBMB Life 53 (1): 3–14. January 2002. doi:10.1080/15216540210811. PMID 12018405.
- "Eps8 in the midst of GTPases". The International Journal of Biochemistry & Cell Biology 34 (10): 1178–83. October 2002. doi:10.1016/S1357-2725(02)00064-X. PMID 12127568.
- "Phosphorylation of calmodulin. Functional implications". European Journal of Biochemistry 269 (15): 3619–31. August 2002. doi:10.1046/j.1432-1033.2002.03038.x. PMID 12153558. https://digital.csic.es/bitstream/10261/79981/1/Phosphorylation%20of%20calmodulin.pdf.
- "Functional implication of the interaction between EGF receptor and c-Src". Frontiers in Bioscience 8 (1–3): s28–38. January 2003. doi:10.2741/980. PMID 12456372.
- "The human plasma proteome: history, character, and diagnostic prospects". Molecular & Cellular Proteomics 1 (11): 845–67. November 2002. doi:10.1074/mcp.R200007-MCP200. PMID 12488461.
- "Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage". Cancer Research 63 (1): 1–5. January 2003. PMID 12517767.
- "Androgen receptor and prostate cancer invasion". International Journal of Andrology 26 (1): 21–5. February 2003. doi:10.1046/j.1365-2605.2003.00375.x. PMID 12534934.
- "Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta". Annals of the New York Academy of Sciences 995 (1): 39–47. May 2003. doi:10.1111/j.1749-6632.2003.tb03208.x. PMID 12814937. Bibcode: 2003NYASA.995...39R.
- "Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor". Growth Factors 22 (2): 89–95. June 2004. doi:10.1080/08977190410001700998. PMID 15253384.
- "Active and inactive conformations of the epidermal growth factor receptor". Biochemical Society Transactions 32 (Pt 5): 742–5. November 2004. doi:10.1042/BST0320742. PMID 15494003.
- "Bi-directional signaling between gastrointestinal peptide hormone receptors and epidermal growth factor receptor". Growth Factors 22 (4): 261–8. December 2004. doi:10.1080/08977190412331286900. PMID 15621729.
- "Planning for intracavitary anti-EGFR radionuclide therapy of gliomas. Literature review and data on EGFR expression". Journal of Neuro-Oncology 77 (1): 33–45. March 2006. doi:10.1007/s11060-005-7410-z. PMID 16200342.
- "Epidermal growth factor receptor: a promising therapeutic target for colorectal cancer". Analytical and Quantitative Cytology and Histology 28 (2): 61–8. April 2006. PMID 16637508.
- "Epidermal growth factor receptor abnormalities in lung cancer. Pathogenetic and clinical implications". Annals of Diagnostic Pathology 10 (5): 306–15. October 2006. doi:10.1016/j.anndiagpath.2006.06.011. PMID 16979526.
- "Epidermal growth factor receptor mutations and susceptibility to targeted therapy in lung cancer". Respirology 11 (6): 687–92. November 2006. doi:10.1111/j.1440-1843.2006.00887.x. PMID 17052295.
- "Somatic mutations of the epidermal growth factor receptor and non-small-cell lung cancer". Journal of Medical Genetics 44 (3): 166–72. March 2007. doi:10.1136/jmg.2006.046102. PMID 17158592.
- "PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors". Clinical Cancer Research 13 (2 Pt 1): 378–81. January 2007. doi:10.1158/1078-0432.CCR-06-1992. PMID 17255257.
- "The epidermal growth factor receptor in malignant gliomas: pathogenesis and therapeutic implications". Expert Opinion on Therapeutic Targets 11 (4): 463–72. April 2007. doi:10.1517/14728222.11.4.463. PMID 17373877.
External links
- Epidermal Growth Factor Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P00533 (Human Epidermal growth factor receptor) at the PDBe-KB.
 |