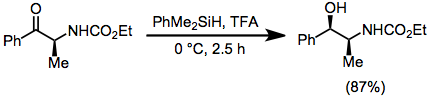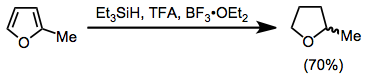Chemistry:Reductions with hydrosilanes
Reductions with hydrosilanes are methods used for hydrogenation and hydrogenolysis of organic compounds. The approach is a subset of ionic hydrogenation. In this particular method, the substrate is treated with a hydrosilane and auxiliary reagent, often a strong acid, resulting in formal transfer of hydride from silicon to carbon.[1] This style of reduction with hydrosilanes enjoys diverse if specialized applications.
Scope
Deoxygenation of alcohols and halides
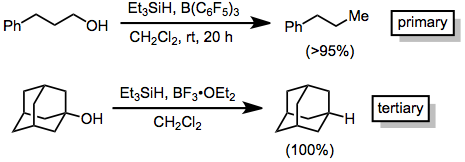
Some alcohols are reduced to alkanes when treated with hydrosilanes in the presence of a strong Lewis acid. Brønsted acids may also be used. Tertiary alcohols undergo facile reduction using boron trifluoride etherate as the Lewis acid.[2] Primary alcohols require an excess of the silane, a stronger Lewis acid, and long reaction times.[3]
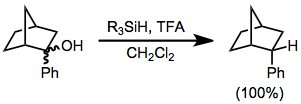
Skeletal rearrangements are sometimes induced.[4] Another side reaction is nucleophilic attack of the conjugate base on the intermediate carbocation.[5] In organosilane reductions of substrates bearing prostereogenic groups, diastereoselectivity is often high. Reduction of either diastereomer of 2-phenyl-2-norbornanol leads exclusively to the endo diastereomer of 2-phenylnorbornane.[6] None of the exo diastereomer was observed.
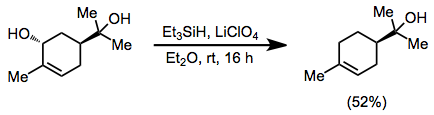
Allylic alcohols may be deoxygenated in the presence of tertiary alcohols when ethereal lithium perchlorate is employed as a source of Li+.[7]
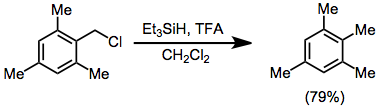
Reductions of alkyl halides and triflates gives poorer yields in general than reductions of alcohols. A Lewis or Bronsted acid is required.[8]
Reduction of carbonyls
- Aldehydes and ketones
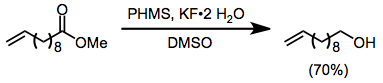
Polymeric hydrosilanes, such as polymethylhydrosiloxane (PHMS), may be employed to facilitate separation of the reduced products from silicon-containing byproducts.[9][10]
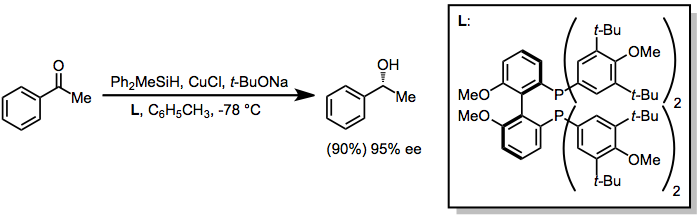
Enantioselective reductions of ketones may be accomplished through the use of catalytic amounts of chiral transition metal complexes.[11] In some cases, the transition metal simply serves as a Lewis acid that coordinates to the ketone oxygen; however, some metals (most notably copper) react with hydrosilanes to afford metal hydride intermediates, which act as the active reducing agent.[12]
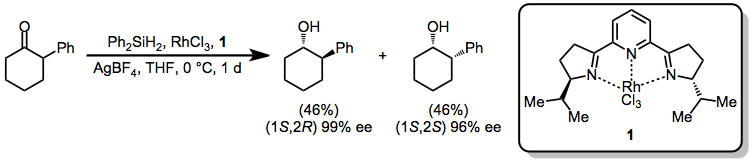
In the presence of rhodium catalyst 1 and rhodium trichloride, 2-phenylcyclohexanone is reduced with no diastereoselectivity but high enantioselectivity.[13]
- Esters
Esters may be reduced to alcohols under conditions of nucleophilic activation with caesium or potassium fluoride.[14]
Aldehydes undergo hydrosilylation in the presence of hydrosilanes and fluoride. The resulting silyl ethers can be hydrolyzed with 1 M hydrochloric acid. Optimal yields of the hydrosilylation are obtained when the reaction is carried out in very polar solvents.[10]
-
[math]\displaystyle{ \ce{\mathit{n}-C10H21CHO} + {\color{Blue}\ce{PhMe2Si}}\ce{H -\gt [\ce{TBAF}][\ce{rt}] \mathit{n}-C10H21CH2O}{\color{Blue}\ce{SiMe2Ph}} }[/math]
()
- [math]\displaystyle{ \begin{array}{lr} \ce{Solvent} & \ce{Yield}(\%) \\ \hline \ce{CH2Cl2} & 1\\ \ce{THF} & 9\\ \ce{Me2NCOH} & 56\\ \ce{DMPU} & 89\\ \ce{HMPA} & 91 \end{array} }[/math]
Reduction of C=C bonds
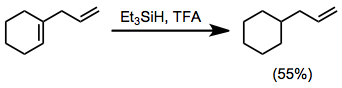
Hydrosilanes can reduce 1,1-disubstituted double bonds that form stable tertiary carbocations upon protonation. Trisubstituted double bonds may be reduced selectively in the presence of 1,2-disubstituted or monosubstituted alkenes.[15]
Aromatic compounds may be reduced with TFA and triethylsilane. Substituted furans are reduced to tetrahydrofuran derivatives in high yield.[16]
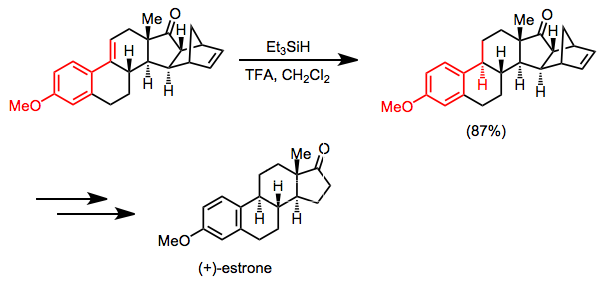
A synthesis of (+)-estrone relies on selective hydrosilane reduction of a conjugated alkene as a key step. The ketone carbonyl and isolated double bond are unaffected under the conditions shown.[17]
Ether cleavage
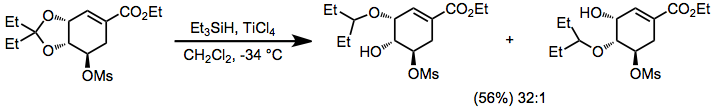
Acetals, ketals, and aminals are reduced in the presence of hydrosilanes and acid. Site-selective reduction of acetals and ketals whose oxygens are inequivalent have been reported—the example below is used in a synthesis of Tamiflu.[18]
Other functional groups that have been reduced with hydrosilanes include amides,[19] and α,β-unsaturated esters[20] enamines,[21] imines,[22] and azides.[23]
Safety
Trifluoroacetic acid, often used in these reductions, is a strong, corrosive acid. Some hydrosilanes are pyrophoric.
References
- ↑ Larson, Gerald L.; Fry, James L. (2008). "Ionic and Organometallic-Catalyzed Organosilane Reductions". Organic Reactions: 1–737. doi:10.1002/0471264180.or071.01. ISBN 978-0471264187.
- ↑ Kraus, George A.; Molina, Maria Teresa; Walling, John A. (1986). "Reduction of cyclic hemiacetals. The synthesis of demethoxyeleutherin and nanaomycin A". Journal of the Chemical Society, Chemical Communications (21): 1568. doi:10.1039/C39860001568.
- ↑ Gevorgyan, Vladimir; Rubin, Michael; Benson, Sharonda; Liu, Jian-Xiu; Yamamoto, Yoshinori (September 2000). "A Novel B(C6F5)3-Catalyzed Reduction of Alcohols and Cleavage of Aryl and Alkyl Ethers with Hydrosilanes †". The Journal of Organic Chemistry 65 (19): 6179–6186. doi:10.1021/jo000726d. PMID 10987957.
- ↑ Adlington, Merwyn G.; Orfanopoulos, Michael; Fry, James L. (August 1976). "A convenient one-step synthesis of hydrocarbons from alcohols through use of the organosilane-boron trifluoride reducing system". Tetrahedron Letters 17 (34): 2955–2958. doi:10.1016/S0040-4039(01)85498-8.
- ↑ Doyle, Michael P.; McOsker, Charles C. (February 1978). "Silane reductions in acidic media. 10. Ionic hydrogenation of cycloalkenes. Stereoselectivity and mechanism". The Journal of Organic Chemistry 43 (4): 693–696. doi:10.1021/jo00398a039.
- ↑ Carey, Francis A.; Tremper, Henry S. (January 1969). "Carbonium ion-silane hydride transfer reactions. II. 2-Phenyl-2-norbornyl cation". The Journal of Organic Chemistry 34 (1): 4–6. doi:10.1021/jo00838a002.
- ↑ Wustrow, David J.; Smith, William J.; Wise, Lawrence D. (January 1994). "Selective deoxygenation of allylic alcohols and acetates by lithium perchlorate promoted triethylsilane reduction". Tetrahedron Letters 35 (1): 61–64. doi:10.1016/0040-4039(94)88162-6.
- ↑ Barclay, L. R. C.; Sonawane, H. R.; MacDonald, M. C. (15 January 1972). "Sterically Hindered Aromatic Compounds. III. Acid-catalyzed Reactions of 2,4,6-Tri- t -butyl- and 2-Methyl-4,6-di-t-butylbenzyl Alcohols and Chlorides". Canadian Journal of Chemistry 50 (2): 281–290. doi:10.1139/v72-041.
- ↑ Pri-Bar, Ilan; Buchman, Ouri (March 1986). "Homogeneous, palladium-catalyzed, selective hydrogenolysis of organohalides". The Journal of Organic Chemistry 51 (5): 734–736. doi:10.1021/jo00355a029.
- ↑ Jump up to: 10.0 10.1 Fujita, Makoto; Hiyama, Tamejiro (November 1988). "Fluoride ion-catalyzed reduction of aldehydes and ketones with hydrosilanes. Synthetic and mechanistic aspects and an application to the threo-directed reduction of .alpha.-substituted alkanones". The Journal of Organic Chemistry 53 (23): 5405–5415. doi:10.1021/jo00258a003.
- ↑ Larson, Gerald L.; Liberatore, Richard J. (26 July 2021). "Organosilanes in Metal-Catalyzed, Enantioselective Reductions". Organic Process Research & Development: acs.oprd.1c00073. doi:10.1021/acs.oprd.1c00073.
- ↑ Lipshutz, Bruce H.; Noson, Kevin; Chrisman, Will; Lower, Asher (July 2003). "Asymmetric Hydrosilylation of Aryl Ketones Catalyzed by Copper Hydride Complexed by Nonracemic Biphenyl Bis - phosphine Ligands". Journal of the American Chemical Society 125 (29): 8779–8789. doi:10.1021/ja021391f. PMID 12862472.
- ↑ Nishiyama, Hisao; Park, Soon-Bong; Itoh, Kenji (August 1992). "Stereoselectivity in hydrosilylative reduction of substituted cyclohexanone derivatives with chiral rhodium-bis(oxazolinyl)pyridine catalyst". Tetrahedron: Asymmetry 3 (8): 1029–1034. doi:10.1016/S0957-4166(00)86036-X.
- ↑ Corriu, R.J.P.; Perz, R.; Reye, C. (January 1983). "Activation of silicon-hydrogen, silicon-oxygen, silicon-nitrogen bonds in heterogeneous phase". Tetrahedron 39 (6): 999–1009. doi:10.1016/S0040-4020(01)88599-9.
- ↑ Kursanov, D. N.; Parnes, Z. N.; Bolestova, G. I. (May 1968). "Ionic hydrogenation of anthracene and dihydroanthragene with a hydride ion donor". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science 17 (5): 1107. doi:10.1007/BF00910867.
- ↑ Bolestova, G. I.; Parnes, Z. N.; Kursanov, D. N. J. Org. Chem. USSR (Engl. Transl.) 1979, 15, 1129.
- ↑ Takano, Seiichi; Moriya, Minoru; Ogasawara, Kunio (March 1992). "A concise stereocontrolled total synthesis of (+)-estrone". Tetrahedron Letters 33 (14): 1909–1910. doi:10.1016/S0040-4039(00)74175-X.
- ↑ Federspiel, Muriel; Fischer, Rolf; Hennig, Michael; Mair, Hans-Jürgen; Oberhauser, Thomas; Rimmler, Gösta; Albiez, Thomas; Bruhin, Jürg et al. (July 1999). "Industrial Synthesis of the Key Precursor in the Synthesis of the Anti-Influenza Drug Oseltamivir Phosphate (Ro 64-0796/002, GS-4104-02): Ethyl (3 R ,4 S ,5 S )-4,5-epoxy-3-(1-ethyl-propoxy)-cyclohex-1-ene-1-carboxylate". Organic Process Research & Development 3 (4): 266–274. doi:10.1021/op9900176.
- ↑ Selvakumar, Kumaravel; Harrod, John F. (2001). "Titanocene-Catalyzed Coupling of Amides in the Presence of Organosilanes To Form Vicinal Diamines". Angewandte Chemie International Edition 40 (11): 2129–2131. doi:10.1002/1521-3773(20010601)40:11<2129::AID-ANIE2129>3.0.CO;2-2.
- ↑ Ojima, Iwao; Kumagai, Miyoko; Nagai, Yoichiro (May 1976). "Hydrosilylation of α,β-unsaturated nitriles and esters catalyzed by tris (triphenylphosphine)chlororhodium". Journal of Organometallic Chemistry 111 (1): 43–60. doi:10.1016/S0022-328X(00)87057-6.
- ↑ Rosentreter, U. (1985). "Stereoselective Synthesis of all- trans -Isomers of 1,2,3,4-Tetrahydropyridines and Piperidines from Hantzsch-Type 1,4-Dihydropyridines". Synthesis 1985 (2): 210–212. doi:10.1055/s-1985-31160.
- ↑ Loim, N. M. (June 1968). "Ionic hydrogenation of the C=N linkage in azomethynes". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science 17 (6): 1345. doi:10.1007/BF01106312.
- ↑ Chandrasekhar, S.; Chandraiah, L.; Reddy, Ch. Raji; Reddy, M. Venkat (July 2000). "Direct Conversion of Azides and Benzyl Carbamates to t- Butyl Carbamates Using Polymethylhydrosiloxane and Pd-C". Chemistry Letters 29 (7): 780–781. doi:10.1246/cl.2000.780.
 |