Chemistry:Boron trifluoride etherate
From HandWiki
| |
| |
| Names | |
|---|---|
| Other names
Boron Trifluoride Ethyl Ether
Boron Trifluoride Diethyl Etherate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| UN number | 2604 |
| |
| |
| Properties | |
| C4H10BF3O | |
| Molar mass | 141.93 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.15 g cm3 |
| Melting point | −58 °C (−72 °F; 215 K) |
| Boiling point | 126 °C (259 °F; 399 K) |
| Hazards | |
| Main hazards | Flammable, Reacts with water, Corrosive |
| GHS pictograms |     
|
| GHS Signal word | DANGER |
| NFPA 704 (fire diamond) | |
| Flash point | 58.5 °C (137.3 °F; 331.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
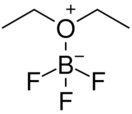
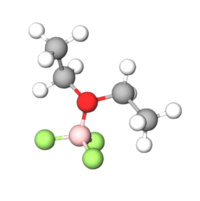
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2. It is a colorless liquid, although older samples can appear brown. The compound is used as a source of boron trifluoride in many chemical reactions that require a Lewis acid.[1] The compound features tetrahedral boron coordinated to a diethylether ligand.[2] Many analogues are known, including the methanol complex.
Reactions
Boron trifluoride etherate serves as a source of boron trifluoride according to the equilibrium:
- BF3OEt2 [math]\ce{ <=>> }[/math] BF3 + OEt2
The BF3 binds to even weak Lewis bases, inducing reactions of the resulting adducts with nucleophiles.[1]
References
- ↑ 1.0 1.1 Veronica Cornel; Carl J. Lovely (2007). "Boron Trifluoride Etherate". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. pp. rb249.pub2. doi:10.1002/047084289X.rb249.pub2. ISBN 978-0-471-93623-7.
- ↑ V. V. Saraev; P. B. Kraikivskii; I. Svoboda; A. S. Kuzakov; R. F. Jordan (2008). "Synthesis, Molecular Structure, and EPR Analysis of the Three-Coordinate Ni(I) Complex [Ni(PPh3)3][BF4]". J. Phys. Chem. A 112 (48): 12449–12455. doi:10.1021/jp802462x. PMID 18991433. Bibcode: 2008JPCA..11212449S.
 |


