Chemistry:Hydrosilane
In chemistry, a hydrosilane is a tetravalent silicon compound containing an Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Especially those compounds that are liquid at room temperature are used in organosilicon chemistry. Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks. Examples include phenylsilane (PhSiH3) and triethoxysilane ((EtO)3SiH).
Synthesis
Hydrosilanes can be prepared by partial hydrosilation of silane itself:
- SiH4 + 3 C2H4 → HSi(C2H5)3
Hydrosilanes classically are prepared by treating chlorosilanes with hydride reagents, such as lithium aluminium hydride:
- 4 ClSi(C2H5)3 + LiAlH4 → 4 HSi(C2H5)3 + "LiAlCl4"
Bonding and structure
The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon (hence the naming convention of silyl hydrides), which results in the polarization of the Si-H bond to be the reverse of that for the C-H bond. Generally silyl hydrides are colourless with physical properties (solubility, volatility) comparable to hydrocarbons. They can be pyrophoric, reflecting the great driving force for replacing Si-H bonds with Si-O bonds.
Reactions and applications
Setting aside silane itself, for which is uned mainly in the microelectronics industry, hydrosilanes participate in many reactions.
Hydrosilylation
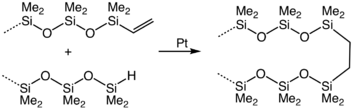
Setting aside silane itself, for which is uned mainly in the microelectronics industry, hydrosilanes participate in hydrosilylation. In this way, the Si-H bond adds across multiple bonds in alkenes, alkynes, imines, and carbonyls. in hydrosilylation. Many organosilicon compounds are prepared in this way. Illustrative is the crosslinking of vinyl-terminated siloxanes:
Hydrolysis
In the presence of platinum-based catalysts, hydrosilanes react with water to give silanols:
- R3SiH + H2O → R3SiOH + H2
Laboratory reductants
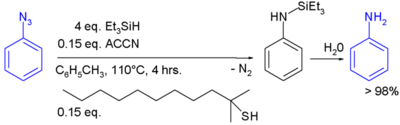
In the laboratory, silyl hydrides are used as reducing agent. For example, PMHS. In one study triethylsilane is used in the conversion of a phenyl azide to an aniline:[1]
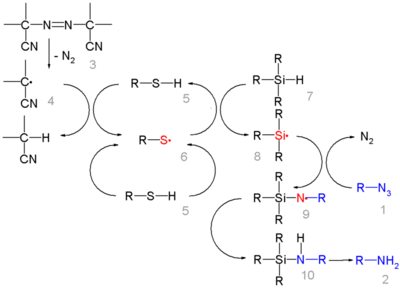
In this reaction ACCN is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle:
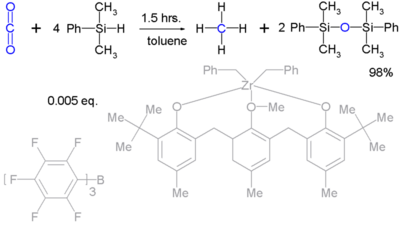
Silyl hydrides can reduce robust molecules such as carbon dioxide (to methane):[2] Unfortunately such reactions are stoichiometric and exclusively of academic interest.
In the related silylmetalation, a metal replaces the hydrogen atom.
Selective reading
- Eulalia Ramírez-Oliva, Alejandro Hernández, J. Merced Martínez-Rosales, Alfredo Aguilar-Elguezabal, Gabriel Herrera-Pérez, and Jorge Cervantesa (2006). Link "Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene". Arkivoc: 126–136. http://-usa.org/ARKIVOC/JOURNAL_CONTENT/manuscripts/2006/EL-1973AP%20as%20published%20mainmanuscript.pdf Link.
References
- ↑ Benati, Luisa; Bencivenni, Giorgio; Leardini, Rino; Minozzi, Matteo; Nanni, Daniele; Scialpi, Rosanna; Spagnolo, Piero; Zanardi, Giuseppe (2006). "Radical Reduction of Aromatic Azides to Amines with Triethylsilane". J. Org. Chem. 71 (15): 5822–5825. doi:10.1021/jo060824k. PMID 16839176.
- ↑ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128, pp 12362 - 12363; doi:10.1021/ja0647250