Chemistry:Reissert indole synthesis
| Reissert indole synthesis | |
|---|---|
| Named after | Arnold Reissert |
| Reaction type | Ring forming reaction |
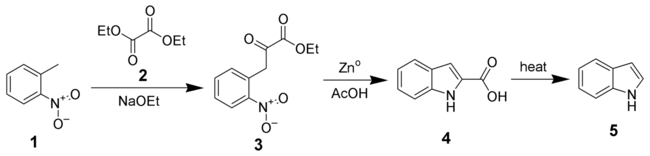
The Reissert indole synthesis is a series of chemical reactions designed to synthesize indole or substituted-indoles (4 and 5) from ortho-nitrotoluene 1 and diethyl oxalate 2.[1][2]
Potassium ethoxide has been shown to give better results than sodium ethoxide.[3]
Reaction mechanism
The first step of the synthesis is the condensation of o-nitrotoluene 1 with a diethyl oxalate 2 to give ethyl o-nitrophenylpyruvate 3. The reductive cyclization of 3 with zinc in acetic acid gives indole-2-carboxylic acid 4. If desired, 4 can be decarboxylated with heat to give indole 5.
Variations
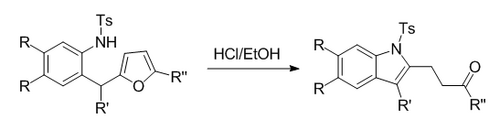
Butin modification
In an intramolecular version of the Reissert reaction, a furan ring-opening provides the carbonyl necessary for cyclization to form an indole. A ketone side chain is present in the final product, allowing further modifications.[4]
See also
- Leimgruber-Batcho indole synthesis
References
- ↑ Reissert, A. (1897). "Einwirkung von Oxalester und Natriumäthylat auf Nitrotoluole. Synthese nitrirter Phenylbrenztraubensäuren". Berichte der deutschen chemischen Gesellschaft 30: 1030–1053. doi:10.1002/cber.189703001200. https://zenodo.org/record/1425858.
- ↑ Noland, W. E.; Baude, F. J. Organic Syntheses, Coll. Vol. 5, p. 567 (1973); Vol. 43, p. 40 (1963). (Article)
- ↑ Johnson, J. R.; Hasbrouck, R. B.; Dutcher, J. D.; Bruce, W. F. (1945). "Gliotoxin. V. The Structure of Certain Indole Derivatives Related to Gliotoxin1,2". J. Am. Chem. Soc. 67 (3): 423. doi:10.1021/ja01219a023.
- ↑ Butin, Alexander; Stroganova, Tatyana; Lodina, Irina; Krapivin, Gennady (2001). "Furan ring opening—indole ring closure: a new modification of the Reissert reaction for indole synthesis". Tetrahedron Letters 42 (10): 2031–3. doi:10.1016/S0040-4039(01)00066-1.
 |