Chemistry:Ritlecitinib
From HandWiki
Short description: Medication
 | |
| Clinical data | |
|---|---|
| Trade names | Litfulo |
| Other names | PF-06651600 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
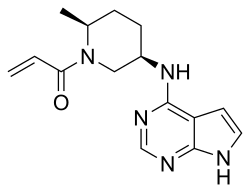
| Formula | C15H19N5O |
| Molar mass | 285.351 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ritlecitinib, sold under the brand name Litfulo, is a medication used for the treatment of severe alopecia areata (hair loss).[1] Ritlecitinib is a kinase inhibitor which inhibits Janus kinase 3 and tyrosine kinase.[1][4][5]
Ritlecitinib was approved for medical use in the United States in June 2023,[1][6] and the European Union in September 2023.[2]
Medical uses
Ritlecitinib is indicated for the treatment of severe alopecia areata for individuals aged 12 and over.[1][2]
Society and culture
Economics
The annual list price of a prescription is $49,000. [7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Litfulo- ritlecitinib capsule". DailyMed. U.S. National Library of Medicine. 23 June 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b2f9446-fb23-4741-b73a-5a2f993733c3.
- ↑ 2.0 2.1 2.2 "Litfulo EPAR". 18 September 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/litfulo.
- ↑ "Litfulo Product information". 18 September 2023. https://ec.europa.eu/health/documents/community-register/html/h1755.htm.
- ↑ "Ritlecitinib". https://drugs.ncats.io/drug/2OYE00PC25.
- ↑ "Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata". Drug Design, Development and Therapy 16: 363–374. February 2022. doi:10.2147/DDDT.S334727. PMID 35210753.
- ↑ "FDA Approves Pfizer's Litfulo (Ritlecitinib) for Adults and Adolescents With Severe Alopecia Areata" (Press release). Pfizer. 23 June 2023. Archived from the original on 25 June 2023. Retrieved 24 June 2023 – via Business Wire.
- ↑ "Pfizer's Litfulo enters the scene in alopecia with adolescent nod to rival Lilly's Olumiant". Fierce Pharma. 26 Jun 2023. https://www.fiercepharma.com/pharma/pfizers-litfulo-enters-scene-alopecia-adolescent-nod-rival-lillys-olumiant.
Further reading
- "Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers". The Journal of Allergy and Clinical Immunology 149 (4): 1318–1328. April 2022. doi:10.1016/j.jaci.2021.10.036. PMID 34863853.
- "Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b-3 trial". Lancet (London, England) 401 (10387): 1518–1529. May 2023. doi:10.1016/S0140-6736(23)00222-2. PMID 37062298.
External links
- Clinical trial number NCT03732807 for "PF-06651600 for the Treatment of Alopecia Areata (ALLEGRO-2b/3)" at ClinicalTrials.gov
 |

