Chemistry:Silicon–oxygen tetrahedron
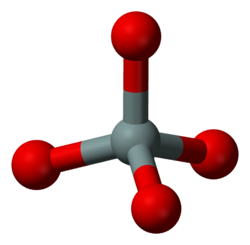
A silicon–oxygen tetrahedron is the SiO4 anionic group, or a silicon atom with four surrounding oxygen atoms arranged to define the corners of a tetrahedron. This is a fundamental component of most silicates in the Earth's crust. A variety of silicate minerals can be identified by the way that the tetrahedra links differ, also by the cations present in the mineral.[1]
Silicon–oxygen tetrahedra links
There are five common silicon–oxygen tetrahedra links:
- Independent tetrahedra
- Single chains
- Double chains
- Sheet silicates
- Framework silicates
Independent tetrahedra
In this group the tetrahedra don't share any oxygen atoms and are independent. Attraction between the tetrahedra and positive ions holds minerals of this structure together. The positive ions could be metal cations such as iron or magnesium. A common mineral in this group is olivine.
Single chains

In single-chain silicates, tetrahedra link to form a chain by sharing two oxygen atoms each. A common mineral in this group is pyroxene.
Double chains

Double-chain silicates occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Sheet silicates
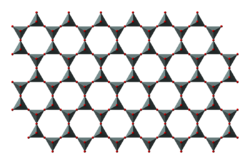
In this group tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and biotite have very weak layers that can be peeled off in sheets.
Framework silicates
In a framework silicate, each tetrahedron shares all 4 oxygen atoms with its neighbours, forming a 3D-4D structure. Quartz and feldspars are in this group.
See also
References
- ↑ Marshak, Stephen (2008). Earth: Portrait of a Planet. New York: W. W. Norton & Company. pp. 135–137. ISBN 978-0-393-93036-8.
