Chemistry:Silyl hydride
Silicon hydrides are organosilicon compounds that contain a silicon–hydrogen bond. Examples include phenylsilane (PhSiH3) and triethoxysilane ((EtO)3SiH).
Bonding and structure
The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon (hence the naming convention of silyl hydrides), which results in the polarization of the Si-H bond to be the reverse of that for the C-H bond. Generally silyl hydrides are colourless with physical properties (solubility, volatility) comparable to hydrocarbons. They can be pyrophoric, reflecting the great driving force for replacing Si-H bonds with Si-O bonds.
Reactions and applications
The dominant application of silicon hydrides is in the production of silicon films and coatings. Silane decomposes as follows:
- SiH4 → Si + 2 H2
This reaction is conducted by chemical vapor deposition, exploits the weakness of the Si-H bond. The second most heavily produced silicon hydride is trichlorosilane, HSiCl3.[1]
Hydrosilylation
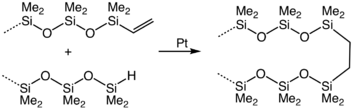
Silyl hydrides adds across multiple bonds in alkenes, alkynes, imines, and carbonyls. in hydrosilylation. Many organosilicon compounds are prepared in this way. Illustrative is the crosslinking of vinyl-terminated siloxanes:
Laboratory reductants
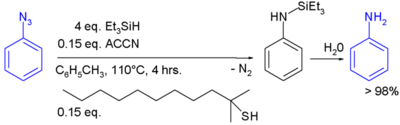
In the laboratory, silyl hydrides are used as reducing agent. For example, PMHS. In one study triethylsilane is used in the conversion of a phenyl azide to an aniline:[2]
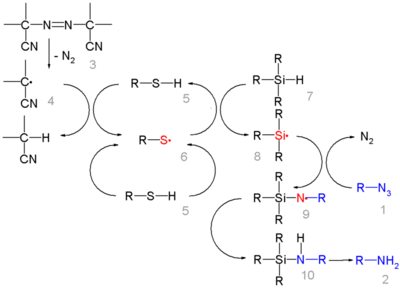
In this reaction ACCN is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle:
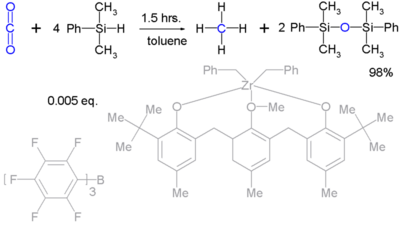
Silyl hydrides can reduce robust molecules such as carbon dioxide (to methane):[3] Unfortunately such reactions are stoichiometric and practical means to regenerate the silicon hydrides are not available.
In the related silylmetalation, a metal replaces the hydrogen atom.
Selective reading
- Eulalia Ramírez-Oliva, Alejandro Hernández, J. Merced Martínez-Rosales, Alfredo Aguilar-Elguezabal, Gabriel Herrera-Pérez, and Jorge Cervantesa (2006). Link "Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene". Arkivoc: 126–136. http://-usa.org/ARKIVOC/JOURNAL_CONTENT/manuscripts/2006/EL-1973AP%20as%20published%20mainmanuscript.pdf Link.
References
- ↑ Simmler, W.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_001.
- ↑ Benati, Luisa; Bencivenni, Giorgio; Leardini, Rino; Minozzi, Matteo; Nanni, Daniele; Scialpi, Rosanna; Spagnolo, Piero; Zanardi, Giuseppe (2006). "Radical Reduction of Aromatic Azides to Amines with Triethylsilane". J. Org. Chem. 71 (15): 5822–5825. doi:10.1021/jo060824k. PMID 16839176.
- ↑ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128, pp 12362 - 12363; doi:10.1021/ja0647250