Chemistry:Sodium-potassium alloy
| Sodium-potassium alloy | |
|---|---|
 Sodium-potassium is liquid at room temperature | |
| Type | metal alloy |
| Physical properties | |
| Density (ρ) |
|
| Thermal properties | |
| Melting temperature (Tm) | −12.6 °C |
| Thermal conductivity (k) at 100 °C | 22.4 W/m⋅K |
| Specific heat capacity (c) | 982 J/kg⋅K |
| Electrical properties | |
| Surface resistivity | 33.5–72.0 µΩ⋅cm |
| Source[1] | |
Sodium-potassium alloy, colloquially called NaK (commonly pronounced /næk/),[2] is an alloy of two alkali metals, sodium (Na, atomic number 11) and potassium (K, atomic number 19), and which is usually liquid at room temperature.[3] Various commercial grades are available. NaK is highly reactive with water (like its constituent elements) and may catch fire when exposed to air, so must be handled with special precautions.
Properties
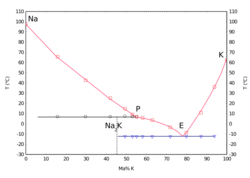
Physical properties
NaK containing 40% to 90% potassium by weight is liquid at room temperature. The eutectic mixture consists of 77% potassium and 23% sodium, is liquid from −12.6 to 785 °C (9.3 to 1,445.0 °F), and has a density of 866 kg/m3 at 21 °C (70 °F) and 855 kg/m3 at 100 °C (212 °F), making it less dense than water.[3] It is highly reactive with water and is stored usually under hexane or other hydrocarbons, or under an inert gas (usually dry nitrogen or argon[5]) if high purity and low levels of oxidation are required.
NaK has a very high surface tension, which makes large amounts of it pull into a bun-like shape. Its specific heat capacity is 982 J/kg⋅K, which is roughly one quarter of that for water, but heat transfer is higher over a temperature gradient due to higher thermal conductivity.[6]
Chemical properties
When stored in air, it forms a yellow potassium superoxide coating and may ignite. This superoxide reacts explosively with water and organics. NaK is not dense enough to sink in most hydrocarbons, but will sink in lighter mineral oil. It is unsafe to store in this manner if the superoxide has formed. A large explosion took place at the Oak Ridge Y-12 facility on December 8, 1999, when NaK cleaned up after an accidental spill and inappropriately treated with mineral oil was scratched with a metal tool.[7] The liquid alloy also attacks PTFE ("Teflon").[8]
Further alloys with low melting points
Further alloys with low melting points are Cs77K23 at −37,5 °C, Cs95Na5 at −30 °C and Na8Rb92 mit −5 °C. The alloy consisting of 40.8 % cesium, 11.8 % sodium and 47.4 % potassium has a melting point of −79.4 °C.
Usage
Coolant
NaK has been used as the coolant in experimental fast neutron nuclear reactors. Unlike commercial plants, these are frequently shut down and defuelled. Use of lead or pure sodium, the other materials used in practical reactors, would require continual heating to maintain the coolant as a liquid. Use of NaK overcomes this. The Dounreay Fast Reactor is an example.
The Soviet RORSAT radar satellites were powered by a BES-5 reactor, which was cooled with NaK.[9][10] In addition to the wide liquid temperature range, NaK has a very low vapor pressure, which is important in the vacuum of space.
An unintended consequence of the usage as a coolant on orbiting satellites has been the creation of additional space debris. NaK coolant has leaked from a number of satellites, including Kosmos 1818 and Kosmos 1867. The coolant self-forms into frozen droplets of solid sodium-potassium of up to several centimeters in size.[11] These solid objects are a source of space debris.[12]
The Danamics LMX Superleggera CPU cooler uses NaK to transport heat from the CPU to its cooling fins.[13]
Desiccant
In contact with water, hydrogen is created.[14] Hence, sodium-potassium alloys are used as desiccants in drying solvents prior to distillation.
Hydraulic fluid
NaK-77, a eutectic alloy of sodium-potassium, can be used as a hydraulic fluid in high-temperature and high-radiation environments, for temperature ranges of 10 to 1,400 °F (−12 to 760 °C). Its bulk modulus at 1,000 °F (538 °C) is 310,000 psi (2.14 GPa), higher than of a hydraulic oil at room temperature. Its lubricity is poor, so positive-displacement pumps are unsuitable and centrifugal pumps have to be used. Addition of caesium shifts the useful temperature range to −95 to 1,300 °F (−71 to 704 °C). NaK-77 alloy was tested in hydraulic and fluidic systems for the Supersonic Low Altitude Missile.[15]
Chemical methods
NaK can be used as catalyst in some reactions, such as the production of ibuprofen.
Synthesis and production
Industrially, NaK is produced in a reactive distillation.[16] In this continuous process, a distillation column is fed with potassium chloride and sodium. In the reaction zone, potassium chloride reacts with sodium to form sodium chloride and potassium. The lighter-boiling potassium is enriched in an upper fractionating zone and drawn at the column head while molten sodium chloride is withdrawn from the bottom.
See also
References
- ↑ Foust, O. J.; United States Atomic Energy Commission (1976). Sodium-NaK engineering handbook. New York: Gordon & Breach. ISBN 978-0-677-03030-2. https://www.osti.gov/servlets/purl/4631555. Retrieved 27 June 2018.
- ↑ Houghton, Rick, Emergency Characterization of Unknown Materials , CRC Press, 2007, p.89
- ↑ 3.0 3.1 "Sodium-Potassium Alloy (NaK)". BASF. http://www.basf.com/inorganics/pdfs/tech_datasheet/NaK.pdf.
- ↑ G.L.C.M. van Rossen, H. van Bleiswijk: Über das Zustandsdiagramm der Kalium-Natriumlegierungen, in: Z. Anorg. Chem., 1912, 74, S. 152–156.
- ↑ Strem Chemical. "MSDS". http://www.strem.com/catalog/v/19-1910/54/potassium_11135-81-2.
- ↑ "Danamics LM10 - Liquid metal put to the test". NordicHardware. 2008-12-04. p. 2. http://www.nordichardware.com/Reviews/?skrivelse=549&page=2.
- ↑ "Y-12 NaK Accident Investigation". U.S. Department of Energy. February 2000. Archived from the original on 2010-05-28. https://web.archive.org/web/20100528034133/http://www.hss.energy.gov/csa/csp/aip/accidents/typea/9912y12/.
- ↑ Klinkrad, Heiner (October 2009). Liquid-metals Handbook. p. 97. https://books.google.com/books?id=6vHjYe2pBdsC.
- ↑ "Old Nuclear-Powered Soviet Satellite Acts Up". 15 January 2009. http://www.space.com/6322-nuclear-powered-soviet-satellite-acts.html.
- ↑ Klinkrad, Heiner (2006-02-23). Space debris: models and risk analysis. p. 83. ISBN 978-3-540-25448-5. https://books.google.com/books?id=EqD4h59KUXgC&pg=PA83.
- ↑ C. Wiedemann et al, "Size distribution of NaK droplets for MASTER-2009", Proceedings of the 5th European Conference on Space Debris, 30 March-2 April 2009, (ESA SP-672, July 2009)
- ↑ A. Rossi et al, "Effects of the RORSAT NaK Drops on the Long Term Evolution of the Space Debris Population" , University of Pisa, 1997.
- ↑ "Danamics LMX Superleggera Cooler Review". 14 May 2010. http://www.bit-tech.net/hardware/cooling/2010/05/14/danamics-lmx-superleggera-review/1.
"Danamics LMX Superleggera review - Liquid Metal?". 8 June 2010. http://www.guru3d.com/articles_pages/danamics_lmx_superleggera_review,2.html. - ↑ Klell, Manfred; Eichlseder, Helmut; Trattner, Alexander (2018), "Speicherung und Transport", Wasserstoff in der Fahrzeugtechnik (Springer Fachmedien Wiesbaden): pp. 109–139, ISBN 978-3-658-20446-4, http://dx.doi.org/10.1007/978-3-658-20447-1_5, retrieved 2020-06-10
- ↑ Controlled Bombs and Guided Missiles of the World War II and Cold War Eras 2002
- ↑ Jackson, C. B.; Werner, R. C. (1957-01-01). "18". The Manufacture of Potassium and NaK. Advances in Chemistry. 19. pp. 169–173. doi:10.1021/ba-1957-0019.ch018. ISBN 9780841221666.

