Chemistry:Sodium triethylborohydride
From HandWiki
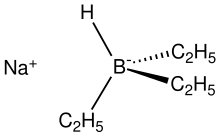
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H16BNa | |
| Molar mass | 121.99 |
| Appearance | white solid |
| Melting point | 30 °C |
| Hazards | |
| Main hazards | pyrophoric |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H261, H314 | |
| P231+232, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P402+404, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium triethylborohydride is an organoboron compound with the formula NaBH(C2H5)3. It is a colorless, pyrophoric solid that is commercially available in toluene solution, unlike the related LiBH(C2H5)3 which is typically sold as a THF solution.[1] It is commonly used for the reductive activation of homogeneous catalysts, converting metal halides to hydrides. Sodium triethylborohydride has been prepared by treating a hot toluene slurry of sodium hydride with triethylborane.[2] The trimethylborohydride analogue, which is assumed to be structurally similar to the triethylborohydride, adopts a tetrameric structure in toluene solution.[3] NaBHEt3 forms a dimeric adduct with tmeda.[4]
References
- ↑ "Callery Borane Products | Callery.com | Callery" (in en-us). 2017-03-05. http://www.callery.com/products/boranes.
- ↑ Binger, P.; Köster, R., "Sodium triethylhydroborate, sodium tetraethylborate, and sodium triethyl-1-propynylborate", Inorg. Synth. 1974, 15, 136-141. doi:10.1002/9780470132463.ch31
- ↑ Bell, N. A.; Coates, G. E.; Heslop, J. A., "Sodium hydridotrimethylboronate and its ether solvate. Study of hydridotrialkylboronates as reagents for the preparation of beryllium hydrides", J. Organomet. Chem. 1987, volume 329, 287-291. doi:10.1016/0022-328X(87)80062-1.
- ↑ Haywood, Joanna; Wheatley, Andrew E. H. (2009). "Metal-Hydride Bonding in Higher Alkali Metal Boron Monohydrides". European Journal of Inorganic Chemistry 2009 (33): 5010–5016. doi:10.1002/ejic.200900756.
 |

