Chemistry:Stemmadenine
From HandWiki
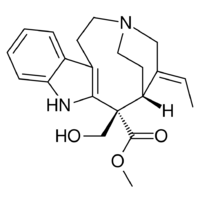
| |
| Names | |
|---|---|
| IUPAC name
Methyl (19E)-17-hydroxy-2,7,19,20-tetradehydro-3,7-seco-15β-curan-16-carboxylate
| |
| Systematic IUPAC name
Methyl (5E,6R,7S)-5-ethylidene-7-(hydroxymethyl)-1,4,5,6,7,8-hexahydro-2H-3,6-ethanoazonino[5,4-b]indole-7-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H26N2O3 | |
| Molar mass | 354.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine.[1] The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways[2][3][4] such as in Aspidosperma species.[5] Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are:
- Catharanthine and Tabersonine in Catharanthus roseus[4][2]
- Subincanadines D-F in Aspidosperma subincanum[3]
It is also present as product in plant like in Tabernaemontana dichotoma seeds.[6]
Pharmacology
It has hypotensive and weak muscle relaxant properties.[6]
See also
References
- ↑ Scott, Alastair I; Qureshi, Asaf A (1969). "Biogenesis of Strychnos, Aspidosperma, and Iboga alkaloids. Structure and reactions of preakuammicine". Journal of the American Chemical Society 91 (21): 5874–6. doi:10.1021/ja01049a032. PMID 5812148.
- ↑ 2.0 2.1 "Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine". Proceedings of the National Academy of Sciences of the United States of America 115 (12): 3180–3185. March 2018. doi:10.1073/pnas.1719979115. PMID 29511102. Bibcode: 2018PNAS..115.3180Q.
- ↑ 3.0 3.1 "The indole-based subincanadine alkaloids and their biogenetic congeners". The Alkaloids. Chemistry and Biology 83: 187–223. 2020. doi:10.1016/bs.alkal.2019.12.001. ISBN 9780128209813. PMID 32098650.
- ↑ 4.0 4.1 "Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine". Biotechnology Letters 26 (10): 793–8. May 2004. doi:10.1023/b:bile.0000025879.53632.f2. PMID 15269549.
- ↑ "Aspidosperma species: A review of their chemistry and biological activities". Journal of Ethnopharmacology 231: 125–140. March 2019. doi:10.1016/j.jep.2018.10.039. PMID 30395977.
- ↑ 6.0 6.1 "Muscle relaxant activity and hypotensive activity of some Tabernaemontana alkaloids". Journal of Ethnopharmacology 13 (2): 165–73. May 1985. doi:10.1016/0378-8741(85)90004-2. PMID 4021514.
 |

