Chemistry:Catharanthine
| |
| Names | |
|---|---|
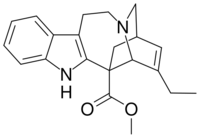
| IUPAC name
Methyl 3,4-didehydroibogamine-18-carboxylate
| |
| Systematic IUPAC name
Methyl (6R,6aR,9S)-7-ethyl-9,10,12,13-tetrahydro-5H-6,9-methanopyrido[1′,2′:1,2]azepino[4,5-b]indole-6(6aH)-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H24N2O2 | |
| Molar mass | 336.435 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Catharanthine is a terpene indole alkaloid produced by the medicinal plant Catharanthus roseus and Tabernaemontana divaricata. Catharanthine is derived from strictosidine, but the exact mechanism by which this happens is currently unknown. Catharanthine is one of the two precursors that form vinblastine, the other being vindoline.
Pharmacology
(+)-Catharanthine competitively inhibits α9α10 nAChRs with potencies higher than that at α3β4 and α4β2 nAChRs and directly blocks CaV2.2.[1] Catharanthine alkaloids are non competitive antagonist of muscle type nAChRs and this is thought to be the case due to presence of catharanthine moiety in those compounds.[2] In in vitro study, it increased the levels of cAMP by inhibiting cAMP phosphodiesterase in brain.[3] It is a potent inhibitor of TRPM8, similar to BCTC.[4] Structural analysis of catharanthine shows activity on TRPM8, TRPA1, and butyrylcholinesterase.[5]
See also
References
- ↑ "Coronaridine congeners decrease neuropathic pain in mice and inhibit α9α10 nicotinic acetylcholine receptors and CaV2.2 channels". Neuropharmacology 175: 108194. September 2020. doi:10.1016/j.neuropharm.2020.108194. PMID 32540451.
- ↑ "Catharanthine alkaloids are noncompetitive antagonists of muscle-type nicotinic acetylcholine receptors". Neurochemistry International 57 (2): 153–61. September 2010. doi:10.1016/j.neuint.2010.05.007. PMID 20493225.
- ↑ "Ethnobotany and ethnopharmacology of Tabernaemontana divaricata". https://www.thefreelibrary.com/Ethnobotany+%26+ethnopharmacology+of+Tabernaemontana+divaricata.-a0181406119.
- ↑ "Identification of Indole Alkaloid Structural Units Important for Stimulus-Selective TRPM8 Inhibition: SAR Study of Naturally Occurring Iboga Derivatives". Journal of Natural Products 77 (8): 1831–8. August 2014. doi:10.1021/np500235b. PMID 25052206.
- ↑ "Catharanthine". http://www.swisstargetprediction.ch/result.php?job=1306790403&organism=Homo_sapiens.
External links
- Jadhav, A.; Liang, W.; Papageorgiou, P. C.; Shoker, A.; Kanthan, S. C.; Balsevich, J.; Levy, A. S.; Heximer, S. et al. (2013). "Catharanthine dilates small mesenteric arteries and decreases heart rate and cardiac contractility by inhibition of voltage-operated calcium channels on vascular smooth muscle cells and cardiomyocytes". The Journal of Pharmacology and Experimental Therapeutics 345 (3): 383–392. doi:10.1124/jpet.112.199661. PMID 23532933.
 |
