Chemistry:TEX-explosive
| |
| |
| Names | |
|---|---|
| IUPAC name
4,10-Dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane
| |
| Identifiers | |
| Properties | |
| C6H6N4O8 | |
| Molar mass | 262.136 g/mol |
| Appearance | colourless solid |
| Density | 1.985 g/cm3, solid |
| Explosive data | |
| Shock sensitivity | Low |
| Friction sensitivity | Low |
| Detonation velocity | 8500 m/s |
| RE factor | 1.70 |
| Hazards | |
| 252 °C (486 °F; 525 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
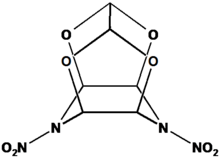
4,10-Dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane - which is most commonly and conveniently abbreviated TEX - is a dense (ρ = 1.985 g cm−3)nitramine high explosive, that derives from the very powerful and sensitive high explosive CL-20. Though related to CL-20 in that is shares the same cage structure TEX is more easily synthesized in good yield from cheap starting materials.[1] Much unlike CL-20 TEX is friction insensitive, bears a low impact sensitivity and possesses a very low shock sensitivity and large critical diameter making it an interesting explosive filler for insensitive munitions.[2] Its systematic name, 4,10-Dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane derives from its tetracyclic structure which is depicted below.
Synthesis and Production
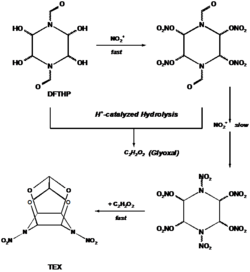
Unlike CL-20, which requires a cumbersome and even costly procedure, TEX is obtained in moderate yield (40 wt.-%) in a one-pot synthesis from 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine (DFTHP) and mixed acid (H2SO4/HNO3). The DFTHP thereby partly undergoes proton-catalyzed hydrolysis and yields glyoxal which reacts as with an intermediate to give TEX as is depicted in the scheme 1.[2]
Performance
Based on the Kamlet Jacobs method the formal idealistic detonation of TEX according to eq. 1.
(1) C6H6N4O8 —→ 3 H2O(g) + 2CO2 + CO + 3 C(gr) + 2 N2
at the ambient temperature density of TEX (1.985 g cm-3) yields a detonation velocity of 8170 m/s and a CJ pressure of 31.4 GPa. Calculations with advanced computer codes like EXPLO and CHEETAH call for even higher detonation velocity but about similar values for the detonation pressure definitely superseding insensitive high explosive NTO. Experimental determination with plastic bonded formulations at high theoretical maximum density exceed the predicted detonation pressures but fall a little short with regards to the detonation velocity if the charge diameter is below 90 mm.[2]
Sensitivity
TEX is not friction sensitive and requires some 40 Joules energy to react in the BAM impact tester. In the autoignition test it yields a mild burn response at 252 °C onset temperature. TEX is also mildly shock sensitive in the Large Scale Gap Test (LSGT).[2]
Toxicity
TEX has a similar water solubility as RDX and hence will be equally mobile in soil and groundwater. However in comparison to insensitive explosive NTO which is highly soluble in water (16 g/l) it should pose a lower level of concern when considering the environmental effects of unexploded or partially exploded charges. Preliminary investigation of the effects of TEX on daphnia and cell cultures show slightly lower toxicity than RDX.[2]
Application
Though known since 1990 [3] TEX is still an experimental explosive. However given its large critical diameter and low shock sensitivity it is an ideal candidate for insensitive large calibre ammunition such as general purpose bombs, artillery shells, torpedo- and depth charges.
See also
- Insensitive Munitions
- RE factor
References
- ↑ A. T. Nielsen Polycyclic Amine Chemistry, in G. A. Olah, D. R. Squire, Chemistry of Energetic Materials (Eds.) Academic Press, 1991, p. 110-111
- ↑ 2.0 2.1 2.2 2.3 2.4 Koch, Ernst-Christian (2015). "TEX - 4,10-Dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane - Review of a Promising High Density Insensitive Energetic Material". Propellants, Explosives, Pyrotechnics 40 (3): 374–387. doi:10.1002/prep.201400195. ISSN 0721-3115.
- ↑ H. Boyer, Joseph; T. Ramakrishnan, Vayalakkavoor; Vedachalam, Murugappa (1990). "4,10-Dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane". Heterocycles 31 (3): 479. doi:10.3987/COM-89-5192. ISSN 0385-5414.
