Chemistry:Glyoxal
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxaldehyde | |||
| Systematic IUPAC name
Ethanedial | |||
| Other names
Glyoxal
Oxalaldehyde | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H2O2 | |||
| Molar mass | 58.036 g·mol−1 | ||
| Density | 1.27 g/cm3 | ||
| Melting point | 15 °C (59 °F; 288 K) | ||
| Boiling point | 51 °C (124 °F; 324 K) | ||
| Thermochemistry | |||
Heat capacity (C)
|
1.044 J/(K·g) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −4 °C (25 °F; 269 K) | ||
| 285 °C (545 °F; 558 K) | |||
| Related compounds | |||
Related aldehydes
|
acetaldehyde glycolaldehyde propanedial methylglyoxal | ||
Related compounds
|
glyoxylic acid glycolic acid oxalic acid pyruvic acid diacetyl acetylacetone | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
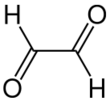
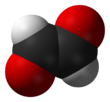
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.[1]
Pure glyoxal is not commonly encountered because it forms hydrates, which oligomerize. For many purposes, these hydrated oligomers behave equivalently to glyoxal. It is produced industrially as a precursor to many products.[2]
Production
Glyoxal was first prepared and named by the German-British chemist Heinrich Debus (1824–1915) by reacting ethanol with nitric acid.[3][4]
Commercial glyoxal is prepared either by the gas-phase oxidation of ethylene glycol in the presence of a silver or copper catalyst (the Laporte process) or by the liquid-phase oxidation of acetaldehyde with nitric acid.[2]
The first commercial glyoxal source was in Lamotte, France, started in 1960. The single largest commercial source is BASF in Ludwigshafen, Germany , at around 60,000 tons per year. Other production sites exist also in the US and China. Commercial bulk glyoxal is made and reported as a 40%-strength solution in water.
Glyoxal may be synthesized in the laboratory by oxidation of acetaldehyde with selenious acid.[5]
Anhydrous glyoxal is prepared by heating solid glyoxal hydrate(s) with phosphorus pentoxide and condensing the vapors in a cold trap.[6]
The experimentally determined Henry's law constant of glyoxal is:
- KH = 4.19 × 105 × exp[62.2 × 103/R × (1/T − 1/298)].[7][clarification needed]
Applications
Coated paper and textile finishes use large amounts of glyoxal as a crosslinker for starch-based formulations. It condenses with urea to afford 4,5-dihydroxy-2-imidazolidinone, which further reacts with formaldehyde to give the bis(hydroxymethyl) derivative dimethylol ethylene urea, which is used for wrinkle-resistant chemical treatments of clothing, i.e. permanent press.
Glyoxal is used as a solubilizer and cross-linking agent in polymer chemistry.
Glyoxal is a valuable building block in organic synthesis, especially in the synthesis of heterocycles such as imidazoles.[8] A convenient form of the reagent for use in the laboratory is its bis(hemiacetal) with ethylene glycol, 1,4-dioxane-2,3-diol. This compound is commercially available.
Glyoxal solutions can also be used as a fixative for histology, that is, a method of preserving cells for examining them under a microscope.
Glyoxal and its derivatives are also used in the chemical probing of RNA structure, as they react with free guanines in RNAs.[9]
Speciation in solution
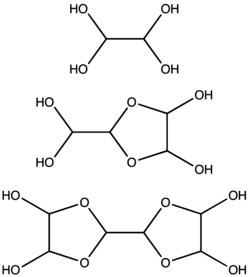
Glyoxal is supplied typically as a 40% aqueous solution.[2] Like other small aldehydes, glyoxal forms hydrates. Furthermore, the hydrates condense to give a series of oligomers, some of which remain of uncertain structure. For most applications, the exact nature of the species in solution is inconsequential. At least one hydrate of glyoxal is sold commercially, glyoxal trimer dihydrate: [(CHO)2]3(H2O)2 (CAS 4405-13-4). Other glyoxal equivalents are available, such as the ethylene glycol hemiacetal 1,4-dioxane-trans-2,3-diol (CAS 4845-50-5, m.p. 91–95 °C),
It is estimated that, at concentrations less than 1 M, glyoxal exists predominantly as the monomer or hydrates thereof, i.e., OCHCHO, OCHCH(OH)2, or (HO)2CHCH(OH)2. At concentrations above 1 M, dimers predominate. These dimers are probably dioxolanes, with the formula [(HO)CH]2O2CHCHO.[10] Dimer and trimers precipitate as solids from cold solutions.
Other occurrences
Glyoxal has been observed as a trace gas in the atmosphere, e.g. as an oxidation product of hydrocarbons.[11] Tropospheric concentrations of 0–200 ppt by volume have been reported, in polluted regions up to 1 ppb by volume.[12]
Safety
The LD50 (oral, rats) is 3300 mg/kg, which is very high.[2]
References
- ↑ O'Neil, M.J. (2001): The Merck Index, 13th Edition, page 803.
- ↑ 2.0 2.1 2.2 2.3 Mattioda, Georges; Blanc, Alain. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_491.pub2.
- ↑ See:
- H. Debus (1857) "On the action of nitric acid on alcohol at common temperatures," Philosophical Magazine, 4th series, 13 : 39–49. From p. 40 : "This residue consisted almost entirely of the aldehyde of glyoxylic acid ; I proposed to call it Glyoxal, C2H4O3."
- H. Debus (1857) "On glyoxal," Philosophical Magazine, 4th series, 13 : 66.
- ↑ Henry Enfield Roscoe and Carl Schorlemmer, A Treatise on Chemistry, vol. 3 (New York, New York: D. Appleton and Co., 1890), pp. 101-102.
- ↑ Ronzio, A. R.; Waugh, T. D. (1944). "Glyoxal Bisulfite". Organic Syntheses 24: 61. http://www.orgsyn.org/demo.aspx?prep=cv3p0438.; Collective Volume, 3, pp. 438
- ↑ Harries, C.; Temme, F. (1907). "Über monomolekulares und trimolekulares Glyoxal". Berichte 40 (1): 165–172. doi:10.1002/cber.19070400124. https://zenodo.org/record/1426217. "Man erhitzt nun das Glyoxal-Phosphorpentoxyd-Gemisch mit freier Flamme und beobachtet bald, dass sich unter Schwarzfärbung des Kolbeninhalte ein flüchtiges grünes Gas bildet, welches sich in der gekühlten Vorlage zu schönen Krystallen von gelber Farbe kondensiert. [One heats the mixture of (crude) glyoxal and P4O10 with an open flame and soon observes, upon blackening of the contents, a mobile green gas which condenses in the cooled flask as beautiful yellow crystals.]".
- ↑ Ip, H. S.; Huang, X. H.; Yu, J. Z. (2009). "Effective Henry's law constants of glyoxal, glyoxylic acid, and glycolic acid". Geophys. Res. Lett. 36 (1): L01802. doi:10.1029/2008GL036212. Bibcode: 2009GeoRL..36.1802I.
- ↑ Snyder, H. R.; Handrick, R. G.; Brooks, L. A. (1942). "Imidazole". Organic Syntheses 22: 65. http://www.orgsyn.org/demo.aspx?prep=cv3p0471.; Collective Volume, 3, pp. 471
- ↑ Mitchell, D; Ritchey, L; Park, H; Babitzke, P; Assmann, S; Bevilacqua, P (2017). "Glyoxals as In Vivo RNA Structural Probes of Guanine Base Pairing". RNA 24: 114–124. doi:10.1261/rna.064014.117.
- ↑ Whipple, E. B. (1970). "Structure of Glyoxal in Water". J. Am. Chem. Soc. 92 (24): 7183–7186. doi:10.1021/ja00727a027.
- ↑ Vrekoussis, M.; Wittrock, F.; Richter, A.; Burrows, J. P. (2009). "Temporal and spatial variability of glyoxal as observed from space". Atmos. Chem. Phys. 9 (13): 4485–4504. doi:10.5194/acp-9-4485-2009.
- ↑ Volkamer, Rainer (2007). "A missing sink for gas‐phase glyoxal in Mexico City: Formation of secondary organic aerosol". Geophys. Res. Lett. 34 (19): 19. doi:10.1029/2007gl030752. Bibcode: 2007GeoRL..3419807V.
External links
- "Glyoxal Industrial Applications". BASF. http://www.intermediates.basf.com/en/intermed/products/glyoxal/application/application-glyoxal.htm.