Chemistry:THIQ
From HandWiki
Short description: Chemical compound
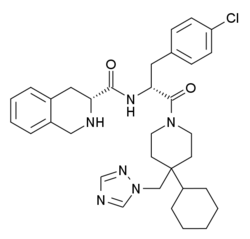 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C33H41ClN6O2 |
| Molar mass | 589.169 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
THIQ is a drug used in scientific research, which is the first non-peptide agonist developed that is selective for the melanocortin receptor subtype MC4.[1][2] In animal studies, THIQ stimulated sexual activity in rats,[3] but with little effect on appetite or inflammation.[4] This supports possible application of MC4 selective agonists for the treatment of sexual dysfunction in humans,[5] although THIQ itself has poor oral bioavailability and a short duration of action so improved analogues will need to be developed.[6]
See also
References
- ↑ "New substituted piperazines as ligands for melanocortin receptors. Correlation to the X-ray structure of "THIQ"". Journal of Medicinal Chemistry 47 (18): 4613–26. August 2004. doi:10.1021/jm0311285. PMID 15317471.
- ↑ "Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists". Biochemistry 44 (34): 11329–41. August 2005. doi:10.1021/bi0501840. PMID 16114870.
- ↑ "Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula". European Journal of Pharmacology 454 (1): 71–9. November 2002. doi:10.1016/S0014-2999(02)02479-2. PMID 12409007.
- ↑ "Functional evaluation of THIQ, a melanocortin 4 receptor agonist, in models of food intake and inflammation". Basic & Clinical Pharmacology & Toxicology 101 (6): 416–20. December 2007. doi:10.1111/j.1742-7843.2007.00133.x. PMID 18028105.
- ↑ "Melanocortins in the treatment of male and female sexual dysfunction". Current Topics in Medicinal Chemistry 7 (11): 1137–44. 2007. doi:10.2174/156802607780906681. PMID 17584134.
- ↑ "Melanocortin receptors, melanotropic peptides and penile erection". Current Topics in Medicinal Chemistry 7 (11): 1098–1106. 2007. doi:10.2174/1568026610707011111. PMID 17584130.
 |
