Chemistry:Tautomycetin
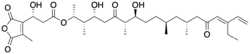 Structure of Tautomycetin | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C33H50O10 |
| Molar mass | 606.753 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tautomycetin is a natural product first isolated from Streptomyces griseochromogenes, a bacterium found in the soil of the Zhejiang Province, China.[1] It was also later found in Penicillium urticae.[2] It is a linear polyketide very similar in structure to tautomycin, both of which contain a unique dialkylmaleic anhydride moiety, which is essential for their pharmacological activity.[3] Tautomycetin is a selective inhibitor of protein phosphatase 1.[4]
Biosynthesis
Much of the biosynthesis of tautomycetin has been deduced.[5] It is synthesized in S. griseochromogenes by two type I polyketide synthases, denoted by modules named TtnA and TtnB. After initial loading with acetyl-CoA, four methylmalonyl-CoAs and three malonyl-CoAs are added alternately by sequential Claisen condensation, followed by one ethylmalonyl-CoA. Each ketone of the incorporated acetate group is selectively modified by a ketoreductase (KR), dehydratase (DH), or enoylreductase (ER) as indicated. The dialkyl maleic anhydride moeity is synthesized separately by eight enzymes (TtnKLMNOPRS) and incorporated into the growing polyketide by esterification mediated via TtnK at the end of TtnA. The polyketide is released from TtnB, by a thioesterase TtnB-TE and undergoes oxidation at the C6 position, catalyzed by the enzyme TtnI. Finally, this intermediate undergoes an enzyme-catalyzed decarboxylation-dehydration to form the final compound, tautomycetin.[citation needed]
The total synthesis of tautomycetin has been reported.[6]
Pharmacology
Tautomycetin has been shown to have antibiotic and antifungal activities.[7] It has also been identified as a potent protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitor with IC50 values as low as 0.21 nM and 0.94 nM respectively, the lowest of over 40 natural product phosphatase inhibitors.[8] In addition, tautomycetin is a potent T cell-specific immunosuppressor [9][10] and has been investigated for treatment in grafting and transplantation.[11] It has useful anticancer properties, inducing apoptosis through the inhibition of several protein cascades in colorectal cancer and thyroid cancer.[12][13][14]
References
- ↑ "A new antibiotic, tautomycetin". The Journal of Antibiotics 42 (1): 141–144. January 1989. doi:10.7164/antibiotics.42.141. PMID 2921220.
- ↑ "Isolation of tautomycetin as a regulator of secondary metabolite production for Penicillium urticae". Bioscience, Biotechnology, and Biochemistry 59 (1): 133–134. January 1995. doi:10.1271/bbb.59.133. PMID 7765963.
- ↑ "The structure of tautomycetin, a dialkylmaleic anhydride antibiotic". The Journal of Antibiotics 43 (7): 890–896. July 1990. doi:10.7164/antibiotics.43.890. PMID 2387780.
- ↑ "PP1:Tautomycetin Complex Reveals a Path toward the Development of PP1-Specific Inhibitors". Journal of the American Chemical Society 139 (49): 17703–17706. December 2017. doi:10.1021/jacs.7b09368. PMID 29156132.
- ↑ "Characterization of the tautomycetin biosynthetic gene cluster from Streptomyces griseochromogenes provides new insight into dialkylmaleic anhydride biosynthesis". Journal of Natural Products 72 (3): 450–459. March 2009. doi:10.1021/np8007478. PMID 19191560.
- ↑ "Synthesis of specific protein phosphatase inhibitors, tautomycin and tautomycetin toward structure-activity relationship study". Current Medicinal Chemistry 9 (22): 2033–2053. November 2002. doi:10.2174/0929867023368818. PMID 12369869.
- ↑ "A new antibiotic, tautomycetin". The Journal of Antibiotics 42 (1): 141–144. January 1989. doi:10.7164/antibiotics.42.141. PMID 2921220.
- ↑ "Tautomycetin is a novel and specific inhibitor of serine/threonine protein phosphatase type 1, PP1". Biochemical and Biophysical Research Communications 287 (2): 328–331. September 2001. doi:10.1006/bbrc.2001.5596. PMID 11554729.
- ↑ "Immunosuppressive effects of tautomycetin in vivo and in vitro via T cell-specific apoptosis induction". Proceedings of the National Academy of Sciences of the United States of America 99 (16): 10617–10622. August 2002. doi:10.1073/pnas.162522099. PMID 12149481. Bibcode: 2002PNAS...9910617S.
- ↑ "T Cell-specific immunosuppression using tautomycetin or PTD-conjugated protein drugs". Yonsei Medical Journal 45 (6): 978–990. December 2004. doi:10.3349/ymj.2004.45.6.978. PMID 15627288.
- ↑ "Tautomycetin as a novel immunosuppressant in transplantation". Transplantation Proceedings 35 (1): 547. February 2003. doi:10.1016/s0041-1345(02)03905-2. PMID 12591525.
- ↑ "Tautomycetin suppresses the TNFalpha/NF-kappaB pathway via inhibition of IKK activation". International Journal of Oncology 33 (5): 1027–1035. November 2008. PMID 18949366.
- ↑ "Effects of tautomycetin on proliferation and fibronectin secretion in vascular smooth muscle cells and glomerular mesangial cells". Transplantation Proceedings 37 (4): 1959–1961. May 2005. doi:10.1016/j.transproceed.2005.02.082. PMID 15919517.
- ↑ "Tautomycetin and tautomycin suppress the growth of medullary thyroid cancer cells via inhibition of glycogen synthase kinase-3beta". Molecular Cancer Therapeutics 8 (4): 914–920. April 2009. doi:10.1158/1535-7163.MCT-08-0712. PMID 19372564.
 |


