Chemistry:Taxadiene
From HandWiki
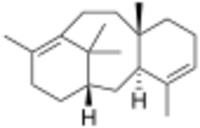
| |
| Names | |
|---|---|
| IUPAC name
Taxa-4,11-diene
| |
| Systematic IUPAC name
(4aS,6S,12aS)-4,9,12a-Trimethyl-1,2,4a,5,6,7,8,11,12,12a-decahydro-6,10-methanobenzo[10]annulene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H32 | |
| Molar mass | 272.476 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Taxadiene (taxa-4,11-diene) is a diterpene. Taxadiene is the first committed intermediate in the synthesis of taxol.[1] Six hydroxylation reactions, and a few others, are needed to convert taxadiene to baccatin III.
Enzymatically, taxadiene is produced from geranylgeranyl pyrophosphate by taxadiene synthase. A biochemical gram-scale production of taxadiene has been reported in 2010 using genetically engineered Escherichia coli.[2]
References
- ↑ Lin, Xiaoyan; Hezari, Mehri; Koepp, Alfred E.; Floss, Heinz G.; Croteau, Rodney (1996). "Mechanism of Taxadiene Synthase, a Diterpene Cyclase That Catalyzes the First Step of Taxol Biosynthesis in Pacific Yew†". Biochemistry 35 (9): 2968–77. doi:10.1021/bi9526239. PMID 8608134.
- ↑ "Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli.". Science 330 (6000): 70–4. 2010. doi:10.1126/science.1191652. PMID 20929806. PMC 3034138. Bibcode: 2010Sci...330...70A. http://dspace.mit.edu/bitstream/1721.1/81256/1/Stephanopoulos_Isoprenoid%20pathway.pdf.
 |

