Chemistry:Baccatin III
From HandWiki
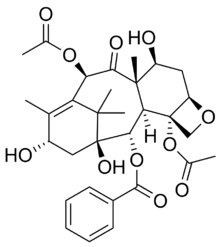
| |
| Names | |
|---|---|
| IUPAC name
(2β,5α,7α,10α,13β)-4,10-Diacetoxy-1,7,13-trihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C31H38O11 | |
| Molar mass | 586.62677 Da |
| Melting point | 229 to 234 °C (444 to 453 °F; 502 to 507 K) |
| Acidity (pKa) | 12.76 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Baccatin III is an isolate from the yew tree (Genera Taxus). Baccatin III is a precursor to the anti-cancer drug paclitaxel (Taxol).
In 2014, researchers reported introduction and expression of the endophytic fungal gene responsible for synthesizing baccatin III (10-deacetylbaccatin III 10-O-acetyltransferase), to the mushroom Flammulina velutipes.[1] Researchers achieved the same accomplishment with Escherichia coli in 2000.[2]
See also
References
- ↑ "Bioproduction of baccatin III, an advanced precursor of paclitaxol with transgenic Flammulina velutipes expressing 10-Deacetylbaccatin III-10-O-acetyl transferase gene.". J Sci Food Agric 94 (12): 2376–2383. 2014. doi:10.1002/jsfa.6562. PMID 24403190.
- ↑ "Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli.". Proc Natl Acad Sci U S A 97 (2): 583–7. 2000. doi:10.1073/pnas.97.2.583. PMID 10639122. Bibcode: 2000PNAS...97..583W.
 |

