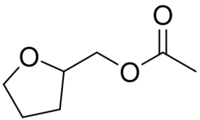
Chemistry:Tetrahydrofurfuryl acetate
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Oxolan-2-yl)methyl acetate | |
| Other names
Tetrahydro-2-furanylmethyl acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| CH3CO2CH2C4H7O | |
| Molar mass | 144.170 g·mol−1 |
| Appearance | clear liquid |
| Density | 1.061 g/cm3 (20 °C) |
| Boiling point | 194 °C (381 °F; 467 K) |
| Miscible | |
| Solubility in alcohol, chloroform, ether | Soluble |
| log P | 0.349 |
Refractive index (nD)
|
1.4475 (liquid 20°) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 83 °C (181 °F; 356 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tetrahydrofurfuryl acetate is an organic chemical compound used for food flavouring and cosmetics. It has a fruity ethereal flavour,[3] also described as honey, maple, or bread-like.[4]
It is generally accepted as safe in the USA.[5] Typical levels of use are 2 ppm in drinks, 8 ppm in ice cream, and 20 ppm in baked products and confectionery.[6]
Classified as a heterocyclic ester, it is made by reacting tetrahydrofurfuryl alcohol with acetic anhydride.[7]
Related flavouring compounds are tetrahydrofurfuryl butyrate, tetrahydrofurfuryl cinnamate, tetrahydrofurfuryl alcohol,[7] and tetrahydrofurfuryl propionate.[8]
References
- ↑ "TETRAHYDROFURFURYL ACETATE | C7H12O3 - PubChem". https://pubchem.ncbi.nlm.nih.gov/compound/tetrahydrofurfuryl_acetate.
- ↑ Burdock, George A. (1997) (in en). Encyclopedia of Food and Color Additives. CRC Press. p. 2754. ISBN 9780849394126. https://books.google.com/books?id=vxtxWneRBN4C&pg=PA2758.
- ↑ Burdock, George A. (1997). Encyclopedia of Food and Color Additives. CRC Press. pp. 2758–. ISBN 9780849394126. https://books.google.com/books?id=vxtxWneRBN4C&pg=PA2758+. Retrieved 17 January 2017.
- ↑ "Food safety and quality: details". Food and Agriculture Organization. http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/1430/. Retrieved 17 January 2017.
- ↑ Administration, Food and Drug; (U.S.), Office of the Federal Register (2001) (in en). The Code of Federal Regulations of the United States of America. U.S. Government Printing Office. p. 54. https://books.google.com/books?id=Vu48AAAAIAAJ&pg=PA54.
- ↑ Food Protection Committee - National Academy of Sciences (1965) (in en). Chemicals Used in Food Processing. National Academies. p. 195. https://books.google.com/books?id=o5ErAAAAYAAJ&pg=PA292.
- ↑ 7.0 7.1 Fenaroli, Giovanni; Furia, Thomas E.; Bellanca, Nicoló (1975) (in en). Fenaroli's Handbook of Flavor Ingredients (2 ed.). Taylor & Francis. p. 529. ISBN 9780878195336. https://books.google.com/books?id=fbnVjdBvFlkC&pg=PA529.
- ↑ Maga, Joseph A.; Katz, Ira (May 1979). "Furans in foods". CRC Critical Reviews in Food Science and Nutrition 11 (4): 355–400. doi:10.1080/10408397909527268. PMID 378551.
 |


