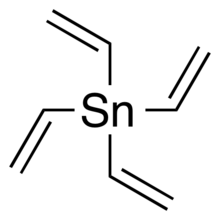
Chemistry:Tetravinyltin
| |
| Names | |
|---|---|
| IUPAC name
tetrakis(ethenyl)stannane
| |
| Other names
tetravinylstannane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C8H12Sn | |
| Molar mass | 226.894 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.246 g/mL |
| Boiling point | 160–163 °C (320–325 °F; 433–436 K) |
| Hazards | |
| Main hazards | Flammable, Toxic |
| Safety data sheet | [1] |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H226, H301, H311, H331 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+310, P302+352, P303+361+353, P304+340, P311, P312, P321, P322, P330, P361, P363, P370+378, P403+233, P403+235 | |
| Flash point | 105 °F |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetravinyltin (also known as tetravinylstannane) is an organotin compound with a chemical formula of C8H12Sn.[1]
Uses
Upon heating, a mixture of tetravinyltin and tin tetrachloride undergo disproportionation to form divinyltin dichloride, vinyltin trichloride, and trivinyltin chloride in high yields.[2] A study about this can be found in the Journal of American Chemical Society.[3]
Tetravinyltin cannot be used for therapeutic or diagnostic purposes and must only be used for research.[4] It can also be used for thin film deposition.[5]
Hazards
According to the European Chemicals Agency, tetravinyltin is flammable in liquid and gas form. It is also toxic when in contact with skin, inhaled, and swallowed.[6] Therefore, personal protective equipment must be used in handling and proper caution applied during use.
References
- ↑ PubChem. "Tetravinyltin" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/66189.
- ↑ "Tetravinyltin | Tetravinylstannane | Sn(CH=CH2)4" (in en-US). https://ereztech.com/tetravinyltin-cas-1112-56-7/.
- ↑ Rosenberg, Sanders D.; Gibbons, Ambrose J. (1957-05-01). "The Disproportionation of Tetravinyltin with Tin Tetrachloride and the Cleavage of Some Vinyltin Compounds with Bromine". Journal of the American Chemical Society 79 (9): 2138–2140. doi:10.1021/ja01566a029. ISSN 0002-7863. https://doi.org/10.1021/ja01566a029.
- ↑ "Tetravinyltin | CAS 1112-56-7" (in en). https://www.scbt.com/p/tetravinyltin-1112-56-7;jsessionid=5mD0QeHZtOrWRqoTDaUufnfdwrWokSs-iS0ZOF7bJeucRjg9nA5-!273371264.
- ↑ Elements, American. "Tetravinyltin" (in en). https://www.americanelements.com/tetravinyltin-1112-56-7.
- ↑ "Tetravinylstannane - Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.012.903.
External links
- TETRAVINYLTIN - ChemicalBook
- Tetravinylstannane at Encyclopedia of Reagents for Organic Synthesis
- Stannane, tetraethenyl at NIST WebBook
 |

