Chemistry:Thiochrome
From HandWiki
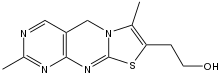
| |
| Names | |
|---|---|
| Other names
2,7-dimethylthiachromine-8-ethanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H14N4OS | |
| Molar mass | 262.33 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.49 g/cm3 |
| Melting point | 228.8 °C (443.8 °F; 501.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiochrome (TChr)is a tricyclic organic compound that arises from the oxidation of the vitamin thiamine. Being highly fluorescent, it is often the derivative that is quantified in the analysis of thiamine. The oxidation can be effected with ceric ammonium nitrate, hydrogen peroxide, and related reagents.[1] Hydrolysis of thiamine can yield a thiol derivative (TSH), which is also susceptible to oxidation to the disulfide (TSST).[2]
References
- ↑ Bettendorff, L.; Wins, P. (2013). "Biochemistry of Thiamine and Thiamine Phosphate Compounds". Encyclopedia of Biological Chemistry. pp. 202–209. doi:10.1016/B978-0-12-378630-2.00102-X. ISBN 9780123786319.
- ↑ Stepuro, I.I. (2005). "Thiamine and vasculopathies". Prostaglandins, Leukotrienes and Essential Fatty Acids 72 (2): 115–127. doi:10.1016/j.plefa.2004.10.009. PMID 15626594.
 |

