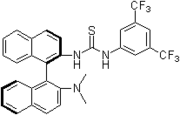
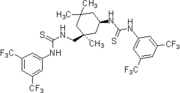
Chemistry:Thiourea organocatalysis
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding ("partial protonation"). The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.[1]
History
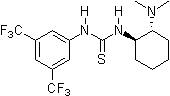
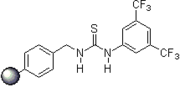
Pioneering contributions were made by Kelly, Etter, Jorgensen, Hine, Curran, Göbel, and De Mendoza (see review articles cited below) on hydrogen bonding interactions of small, metal-free compounds with electron-rich binding sites. Peter R. Schreiner and co-workers identified and introduced electron-poor thiourea derivatives as hydrogen-bonding organocatalysts. Schreiner's thiourea, N,N-bis3,5-bis(trifluormethyl)phenyl thiourea, combines all structural features for double H-bonding mediated organocatalysis:
- electron-poor
- rigid structure
- non-coordinating, electron withdrawing substituents in 3,4, and/or 5 position of a phenyl ring
- the 3,5-bis(trifluoromethyl)phenyl-group is the preferred substituent
Catalyst-substrate interactions
Hydrogen-bonding between thiourea derivatives and carbonyl substrates involve two hydrogen bonds provided by coplanar amino substituents in the (thio)urea.[2][3][4]
[5] Squaramides engage in double H-bonding interactions and are often superior to thioureas.[6]
Thioureas are often found to be stronger hydrogen-bond donors (i.e., more acidic) than ureas[7] because their amino groups are more positively charged. Quantum chemical analyses revealed that this counterintuitive phenomenon, which is not explainable by the relative electronegativities of O and S, results from the effective steric size of the chalcogen atoms.[8]
Advantages of thiourea organocatalysts
Thio) ureas are green and sustainable catalysts. When effective, they can offer these advantages:
- absence of product inhibition due to weak enthalpic binding, but specific binding-“recognition“
- low catalyst-loading (down to 0.001 mol%)[3]
- high TOF (Turn-Over-Frequency) values (up to 5,700 h−1)[3]
- simple and inexpensive synthesis from primary amine functionalized (chiral-pool) starting materials and isothiocyanates
- easy to modulate and to handle (bench-stable), no inert gas atmosphere required
- immobilization on a solid phase (polymer-bound organocatalysts), catalyst recovery and reusability [3]
- catalysis under almost neutral conditions (pka thiourea 21.0) and mild conditions, acid-sensitive substrates are tolerated
- metal-free, nontoxic (compare traditional metal-containing Lewis-acid catalysts)
- water-tolerant, even catalytically effective in water or aqueous media.[10]
Substrates
H-bond accepting substrates include carbonyl compounds, imines, nitroalkenes. The Diels-Alder reaction is one process that can benefit from (thio)urea catalysts.
Catalysts
A broad variety of monofunctional and bifunctional (concept of bifunctionality) chiral double hydrogen-bonding (thio)urea organocatalysts have been developed to accelerate various synthetically useful organic transformations
2004: Nagasawa's chiral bis-thiourea organocatalyst, catalysis of asymmetric Baylis-Hillman reactions.[14]
2005: Nagasawa's bifunctional thiourea functionalized guanidine, asymmetric catalysis of Henry(Nitroaldol)reactions.[15]
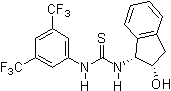
2005: Ricci's chiral thiourea derivative with additional hydroxy-group, enantioselective Friedel-Crafts alkylation of indols with nitroalkenes.[16]
2005: Wei Wang's bifunctional binaphthyl-thiourea derivative, asymmetric catalysis of Morita-Baylis-Hillman reactions.[17]
2006: Yong Tang's chiral bifunctional pyrrolidine-thiourea, enantioselective Michael additions of cyclohexanone to nitroolefins.[20]
2006: Berkessel's chiral isophoronediamine-derived bisthiourea derivative, catalysis of asymmetric Morita-Baylis-Hillman reactions.[21]
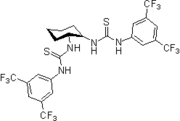
2006: Takemoto's PEG-bound chiral thiourea, asymmetric catalysis of (tandem-) Michael reactions of trans"-β-nitrostyrene, aza-Henry reactions.[22]
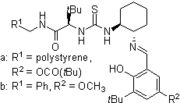
2007: Kotke/Schreiner, polystyrene-bound, recoverable and reusable thiourea derivative for organocatalytic tetrahydropyranylation of alcohols.[3]
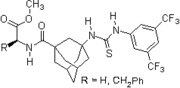
2007: Wanka/Schreiner, chiral peptidic adamantane-based thiourea, catalysis of Morita-Baylis-Hillman reactions.[23]
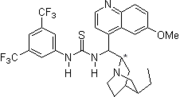
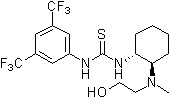
2007: Takemoto's chelating bifunctional hydroxy-thiourea for enantioselective Petasis-type reaction of quinolines.[24]
Further reading
- Christian M. Kleiner, Peter R. Schreiner (2006). "Hydrophobic amplification of noncovalent organocatalysis". Chem. Commun.: 4315–4017.
- Z. Zhang and P. R. Schreiner (2007). "Thiourea-Catalyzed Transfer Hydrogenation of Aldimines". Synlett 2007 (9): 1455–1457. doi:10.1055/s-2007-980349.
- Wanka, Lukas; Chiara Cabrele; Maksims Vanejews; Peter R. Schreiner (2007). "γ-Aminoadamantanecarboxylic Acids Through Direct C–H Bond Amidations". European Journal of Organic Chemistry 2007 (9): 1474–1490. doi:10.1002/ejoc.200600975. ISSN 1434-193X.
References
- ↑ Kotke, Mike; Schreiner, Peter R. (October 2009). "(Thio)urea Organocatalysts". in Petri M. Pihko. Hydrogen Bonding in Organic Synthesis. pp. 141 to 251. ISBN 978-3-527-31895-7. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-352731895X.html.
- ↑ Alexander Wittkopp, Peter R. Schreiner, "Diels-Alder Reactions in Water and in Hydrogen-Bonding Environments", book chapter in "The Chemistry of Dienes and Polyenes" Zvi Rappoport (Ed.), Volume 2, John Wiley & Sons Inc.; Chichester, 2000, 1029-1088. ISBN:0-471-72054-2.
Alexander Wittkopp, "Organocatalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic and Aqueous Solvents", dissertation written in German, Universität Göttingen, 2001. English abstract/download: [1]
Peter R. Schreiner, review: "Metal-free organocatalysis through explicit hydrogen bonding interactions", Chem. Soc. Rev. 2003, 32, 289-296. abstract/download:[2]
M. Kotke and P. R. Schreiner (2006). "Acid-free, organocatalytic acetalization". Tetrahedron 62 (2–3): 434–439. doi:10.1016/j.tet.2005.09.079.M. P. Petri (2004). "Activation of Carbonyl Compounds by Double Hydrogen Bonding: An Emerging Tool in Asymmetric Catalysis". Angewandte Chemie International Edition 43 (16): 2062–2064. doi:10.1002/anie.200301732. PMID 15083451.
Yoshiji Takemoto, review: "Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors", Org. Biomol. Chem. 2005, 3, 4299-4306. abstract/download: [3]Mark S. Taylor, Eric N. Jacobsen (2006). "Asymmetric Catalysis by Chiral Hydrogen-Bond Donors". Angewandte Chemie International Edition 45 (10): 1520–1543. doi:10.1002/anie.200503132. PMID 16491487.J. C. Stephen (2006). "Organocatalysis Mediated by (Thio)urea Derivatives". Chemistry 12 (21): 5418–5427. doi:10.1002/chem.200501076. PMID 16514689. - ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Kotke, Mike; Peter Schreiner (2007). "Generally Applicable Organocatalytic Tetrahydropyranylation of Hydroxy Functionalities with Very Low Catalyst Loading". Synthesis 2007 (5): 779–790. doi:10.1055/s-2007-965917. ISSN 0039-7881.
- ↑ Jump up to: 4.0 4.1 Schreiner, Peter R.; Alexander Wittkopp (2002). "H-Bonding Additives Act Like Lewis Acid Catalysts". Organic Letters 4 (2): 217–220. doi:10.1021/ol017117s. ISSN 1523-7060. PMID 11796054.
- ↑ Kotke, Mike (2009). Hydrogen-Bonding (Thio)urea Organocatalysts in Organic Synthesis : State of the art and Practical Methods for Acetalization, Tetrahydropyranylation, and Cooperative Epoxide Alcoholysis (Ph.D.). University Giessen/Germany. http://geb.uni-giessen.de/geb/volltexte/2010/7835/. Retrieved 2010-11-12.
- ↑ Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. (2015). "Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions". Adv. Synth. Catal. 357 (2–3): 253–281. doi:10.1002/adsc.201401003.
- ↑ Jakab, Gergely; Tancon, Carlo; Zhang, Zhiguo; Lippert, Katharina M.; Schreiner, Peter R. (2012). "(Thio)urea Organocatalyst Equilibrium Acidities in DMSO". Organic Letters 14 (7): 1724–1727. doi:10.1021/ol300307c.
- ↑ Nieuwland, Celine; Fonseca Guerra, Célia (2022). "How the Chalcogen Atom Size Dictates the Hydrogen‐Bond Donor Capability of Carboxamides, Thioamides, and Selenoamides". Chemistry – A European Journal 28 (31): e202200755. doi:10.1002/chem.202200755.
- ↑ Wittkopp, Alexander; Peter R. Schreiner (2003). "Metal-Free, Noncovalent Catalysis of Diels–Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water". Chemistry: A European Journal 9 (2): 407–414. doi:10.1002/chem.200390042. ISSN 0947-6539. PMID 12532289.
- ↑ A. Wittkopp and P. R. Schreiner (2003). "Metal-Free, Noncovalent Catalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water". Chemistry 9 (2): 407–414. doi:10.1002/chem.200390042. PMID 12532289.
- ↑ Sigman, Matthew S.; Eric N. Jacobsen (1998). "Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries". Journal of the American Chemical Society 120 (19): 4901–4902. doi:10.1021/ja980139y. ISSN 0002-7863.
- ↑ Sigman, Matthew S.; Petr Vachal; Eric N. Jacobsen (2000). "A General Catalyst for the Asymmetric Strecker Reaction". Angewandte Chemie International Edition 39 (7): 1279–1281. doi:10.1002/(SICI)1521-3773(20000403)39:7<1279::AID-ANIE1279>3.0.CO;2-U. ISSN 1433-7851. PMID 10767031.
- ↑ Okino, Tomotaka; Yasutaka Hoashi; Yoshiji Takemoto (2003). "Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts". Journal of the American Chemical Society 125 (42): 12672–12673. doi:10.1021/ja036972z. ISSN 0002-7863. PMID 14558791.
- ↑ Sohtome, Yoshihiro; Aya Tanatani; Yuichi Hashimoto; Kazuo Nagasawa (2004). "Development of bis-thiourea-type organocatalyst for asymmetric Baylis–Hillman reaction☆". Tetrahedron Letters 45 (29): 5589–5592. doi:10.1016/j.tetlet.2004.05.137. ISSN 0040-4039.
- ↑ Sohtome, Yoshihiro; Yuichi Hashimoto; Kazuo Nagasawa (2005). "Guanidine-Thiourea Bifunctional Organocatalyst for the Asymmetric Henry (Nitroaldol) Reaction". Advanced Synthesis & Catalysis 347 (11–13): 1643–1648. doi:10.1002/adsc.200505148. ISSN 1615-4150.
- ↑ Herrera, Raquel P.; Valentina Sgarzani; Luca Bernardi; Alfredo Ricci (2005). "Catalytic Enantioselective Friedel-Crafts Alkylation of Indoles with Nitroalkenes by Using a Simple Thiourea Organocatalyst". Angewandte Chemie International Edition 44 (40): 6576–6579. doi:10.1002/anie.200500227. ISSN 1433-7851. PMID 16172992.
- ↑ Wang, Jian; Hao Li; Xinhong Yu; Liansuo Zu; Wei Wang (2005). "Chiral Binaphthyl-Derived Amine-Thiourea Organocatalyst-Promoted Asymmetric Morita−Baylis−Hillman Reaction". Organic Letters 7 (19): 4293–4296. doi:10.1021/ol051822+. ISSN 1523-7060. PMID 16146410. https://figshare.com/articles/Chiral_Binaphthyl_Derived_Amine_Thiourea_Organocatalyst_Promoted_Asymmetric_Morita_Baylis_Hillman_Reaction/3373348.
- ↑ Vakulya, Benedek; Szilárd Varga; Antal Csámpai; Tibor Soós (2005). "Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts". Organic Letters 7 (10): 1967–1969. doi:10.1021/ol050431s. ISSN 1523-7060. PMID 15876031.
- ↑ McCooey, Séamus H.; Stephen J. Connon (2005). "Urea- and Thiourea-Substituted Cinchona Alkaloid Derivatives as Highly Efficient Bifunctional Organocatalysts for the Asymmetric Addition of Malonate to Nitroalkenes: Inversion of Configuration at C9 Dramatically Improves Catalyst Performance". Angewandte Chemie International Edition 44 (39): 6367–6370. doi:10.1002/anie.200501721. ISSN 1433-7851. PMID 16136619.
- ↑ Cao, Chun-Li; Meng-Chun Ye; Xiu-Li Sun; Yong Tang (2006). "Pyrrolidine−Thiourea as a Bifunctional Organocatalyst: Highly Enantioselective Michael Addition of Cyclohexanone to Nitroolefins". Organic Letters 8 (14): 2901–2904. doi:10.1021/ol060481c. ISSN 1523-7060. PMID 16805512.
- ↑ Berkessel, Albrecht; Katrin Roland; Jörg M. Neudörfl (2006). "Asymmetric Morita−Baylis−Hillman Reaction Catalyzed by Isophoronediamine-Derived Bis(thio)urea Organocatalysts". Organic Letters 8 (19): 4195–4198. doi:10.1021/ol061298m. ISSN 1523-7060. PMID 16956185.
- ↑ Miyabe, Hideto; Sayo Tuchida; Masashige Yamauchi; Yoshiji Takemoto (2006). "Reaction of Nitroorganic Compounds Using Thiourea Catalysts Anchored to Polymer Support". Synthesis 2006 (19): 3295–3300. doi:10.1055/s-2006-950196. ISSN 0039-7881.
- ↑ Wanka, Lukas; Chiara Cabrele; Maksims Vanejews; Peter R. Schreiner (2007). "γ-Aminoadamantanecarboxylic Acids Through Direct C–H Bond Amidations". European Journal of Organic Chemistry 2007 (9): 1474–1490. doi:10.1002/ejoc.200600975. ISSN 1434-193X.
- ↑ Yamaoka, Yousuke; Hideto Miyabe; Yoshiji Takemoto (2007). "Catalytic Enantioselective Petasis-Type Reaction of Quinolines Catalyzed by a Newly Designed Thiourea Catalyst". Journal of the American Chemical Society 129 (21): 6686–6687. doi:10.1021/ja071470x. ISSN 0002-7863. PMID 17488015. https://figshare.com/articles/Catalytic_Enantioselective_Petasis_Type_Reaction_of_Quinolines_Catalyzed_by_a_Newly_Designed_Thiourea_Catalyst/3004408.
- ↑ Liu, Kun; Han-Feng Cui; Jing Nie; Ke-Yan Dong; Xiao-Juan Li; Jun-An Ma (2007). "Highly Enantioselective Michael Addition of Aromatic Ketones to Nitroolefins Promoted by Chiral Bifunctional Primary Amine-thiourea Catalysts Based on Saccharides". Organic Letters 9 (5): 923–925. doi:10.1021/ol0701666. ISSN 1523-7060. PMID 17288432.
- ↑ Li, Xiao-Juan; Kun Liu; Hai Ma; Jing Nie; Jun-An Ma (2008). "Highly Enantioselective Michael Addition of Malonates to Nitroolefins Catalyzed by Chiral Bifunctional Tertiary Amine-Thioureas Based on Saccharides". Synlett 2008 (20): 3242–3246. doi:10.1055/s-0028-1087370. ISSN 0936-5214.
 |