Chemistry:Beta-Nitrostyrene
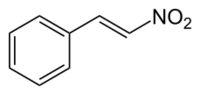
| |
| Names | |
|---|---|
| Preferred IUPAC name
[(E)-2-Nitroethen-1-yl]benzene | |
| Other names
β-Nitro-styrene, 2-Nitrovinylbenzene, 1-Nitro 2-Nitroethenylbenzene, ω-Nitrostyrene, γ-Nitrostyrene, trans-β-nitrostyrene, MFCD00007402
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C8H7NO2 | |
| Molar mass | 149.149 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Melting point | 58 °C (136 °F; 331 K) |
| Boiling point | 255 °C (491 °F; 528 K) |
| Hazards | |
| Safety data sheet | MSDS at Sigma Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
β-Nitrostyrene is an aromatic compound and a nitroalkene used in the synthesis of indigo dye[1] and the slimicide bromo-nitrostyrene.[2]
Applications
β-Nitrostyrene is a chemical precursor for slimicides and dyes. Specifically bromo-nitrostyrene is obtained upon treatment with bromine followed by partial dehydrohalogenation[2] while 2-nitrobenzaldehyde is obtained by treatment with ozone respectively.[1]
Many of the syntheses of psychedelic substituted phenethylamines and substituted amphetamines described by Alexander Shulgin in his book PiHKAL use substituted nitrostyrenes as precursors. They are the final precursor, reduced with lithium aluminium hydride to the final product (an amine).[3]
Chemical synthesis
The chemical is produced by either the Henry reaction of benzaldehyde and nitromethane[4][5] or by direct nitration of styrene using nitric oxide.[6]
References
- ↑ 1.0 1.1 Wright, Elaine; Brühne, Friedrich (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. p. 8. doi:10.1002/14356007.a03_463.
- ↑ 2.0 2.1 Markofsky, Sheldon B. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. p. 6. doi:10.1002/14356007.a17_401.
- ↑ Shulgin, Alexander (1991). Pihkal : a chemical love story. Berkeley, CA: Transform Press. ISBN 978-0-9630096-0-9.
- ↑ Furniss, Brian; Hannaford, Antony; Smith, Peter; Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed.. London: Longman Science & Technical. p. 1035. ISBN 9780582462366. https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd.
- ↑ Worrall, David E. (1929). "Nitrostyrene". Org. Synth. 9: 66. doi:10.15227/orgsyn.009.0066. http://www.orgsyn.org/Content/pdfs/procedures/cv1p0413.pdf. Retrieved 13 January 2014.
- ↑ Mukaiyama, T.; Hata E.; Yamada, T. (1995). "Convenient and Simple Preparation of Nitroolefins Nitration of Olefins with Nitric Oxide". Chemistry Letters 24 (7): 505–506. doi:10.1246/cl.1995.505. https://www.jstage.jst.go.jp/article/cl/24/7/24_7_505/_article. Retrieved 5 January 2014.
 |

