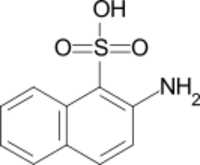
Chemistry:Tobias acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminonaphthalene-1-sulfonic acid | |
| Other names
2-Naphthylamine-1-sulfonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H9NO3S | |
| Molar mass | 223.25 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tobias acid (2-amino-1-naphthalenesulfonic acid) is an organic compound with the formula C10H6(SO3H)(NH2). It is named after the German chemist Georg Tobias.[1] It is one of several aminonaphthalenesulfonic acids, which are derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear otherwise.[2] It is used in the synthesis of azo dyes such as C.I. Acid Yellow 19 and C.I. Pigment Red 49. It is prepared via the Bucherer reaction of 2-hydroxynaphthalene-1-sulfonic acid with ammonia and ammonium sulfite.[3]
References
- ↑ Tobias, Georg (January 1890). "Zur Anwendung der Sandmeyer' schen Reaction auf Diazosulfosäuren und über die Zersetzung dieser Verbindungen bei Gegenwart von Kupfer oder Kupferoxydul" (in German). Berichte der Deutschen Chemischen Gesellschaft 23 (1): 1628–1634. doi:10.1002/cber.189002301270.
- ↑ CID 6670 from PubChem
- ↑ Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_009.
External links
- 2-Amino-1-naphthalenesulfonic acid, NIST Standard Reference Data Program
 |

