Chemistry:Tomaymycin
From HandWiki
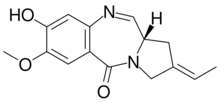
| |
| Names | |
|---|---|
| IUPAC name
(6R,6aS,8Z)-8-ethylidene-3-hydroxy-2,6-dimethoxy-6,6a,7,9-tetrahydro-5H-pyrrolo[2,1-c][1,4]benzodiazepin-11-one[1]
| |
| Other names
GNF-Pf-1072[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H20N2O4 | |
| Molar mass | 304.346 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tomaymycin is an antitumor and antibiotic pyrrolobenzodiazepine with the molecular formula C16H20N2O4.[2][1][3][4] Tomaymycin is produced by the bacterium Streptomyces achromogenes.[2]
References
- ↑ 1.0 1.1 1.2 "Tomaymycin" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Tomaymycin#section=3D-Conformer.
- ↑ 2.0 2.1 Li, Wei; Chou, ShenChieh; Khullar, Ankush; Gerratana, Barbara (May 2009). "Cloning and Characterization of the Biosynthetic Gene Cluster for Tomaymycin, an SJG-136 Monomeric Analog". Applied and Environmental Microbiology 75 (9): 2958–2963. doi:10.1128/AEM.02325-08. PMID 19270147. Bibcode: 2009ApEnM..75.2958L.
- ↑ von Tesmar, Alexander; Hoffmann, Michael; Pippel, Jan; Fayad, Antoine Abou; Dausend-Werner, Stefan; Bauer, Armin; Blankenfeldt, Wulf; Müller, Rolf (October 2017). "Total Biosynthesis of the Pyrrolo[4,2]benzodiazepine Scaffold Tomaymycin on an In Vitro Reconstituted NRPS System". Cell Chemical Biology 24 (10): 1216–1227.e8. doi:10.1016/j.chembiol.2017.08.001. PMID 28890318.
- ↑ Tomaymycin | Antitumor Antibiotic | MedChemExpress. https://www.medchemexpress.com/tomaymycin.html?locale=de-DE?src=google-product&gclid=EAIaIQobChMInZezuKO29wIVhBB9Ch1w0Q9-EAAYASAAEgKRe_D_BwE.
Further reading
- Szeinbach, Sheryl Lynn (1983) (in en). The Genetic Activity of Anthramycin, Tomaymycin and Sibiromycin in Bacterial Forward- and Reverse-mutation Assays and in the Mouse Bone-marrow Micronucleus Test. University of Kentucky.
- Corcoran, John W. (6 December 2012) (in en). Biosynthesis. Springer Science & Business Media. p. 290. ISBN 978-3-642-67724-3.
- Benedetti, Françoise; Perrin, Marc-Antoine; Bosc, Sebastien; Chouteau, Franck; Champion, Nicolas; Bigot, Antony (15 May 2020). "Total Synthesis of (+)-Oxo-tomaymycin". Organic Process Research & Development 24 (5): 762–768. doi:10.1021/acs.oprd.0c00009.
 |

