Chemistry:Kjeldahl method
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation: [ˈkʰelˌtɛˀl]) in analytical chemistry is a method for the quantitative determination of nitrogen contained in organic substances plus the nitrogen contained in the inorganic compounds ammonia and ammonium (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen content and protein content it is an important method for analyzing proteins. This method was developed by Johan Kjeldahl in 1883.[1][2]
Method
The method consists of heating a sample to 360–410 °C with concentrated sulfuric acid (H2SO4), which decomposes ("digests" or "destructs") the organic sample by oxidation to liberate the reduced nitrogen as ammonium sulfate.[3] Hot concentrated sulfuric acid oxidizes carbon (as bituminous coal) and sulfur (see sulfuric acid's reactions with carbon):
- C + 2 H2SO4 → CO2 + 2 SO2 + 2 H2O
- S + 2 H2SO4 → 3 SO2 + 2 H2O
Catalysts like selenium, Hg2SO4 or CuSO4 are often added to make the digestion go faster. Na2SO4 or K2SO4 is also added to increase the boiling point of H2SO4. Digestion is complete when the liquor clarifies with the release of fumes.[3] A distillation system depicted below is built.
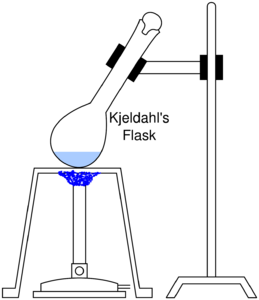 |
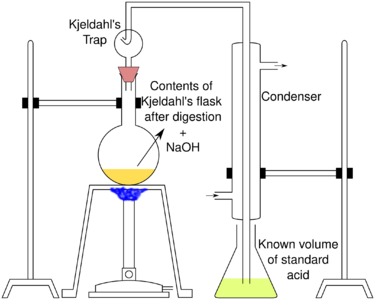
|
The end of the condenser is dipped into a known volume of standard acid (i.e. acid of known concentration). A weak acid like boric acid (H3BO3) in excess of ammonia is often used. Standardized HCl, H2SO4 or some other strong acid can be used instead, but this is less commonplace. The sample solution is then distilled with a small amount of sodium hydroxide (NaOH).[3] NaOH can also be added with a dropping funnel.[4] NaOH reacts the ammonium (NH4+) to ammonia (NH3), which boils off the sample solution. Ammonia bubbles through the standard acid solution and reacts back to ammonium salts with the weak or strong acid.[3]
Ammonium ion concentration in the acid solution, and thus the amount of nitrogen in the sample, is measured via titration. If boric acid (or some other weak acid) was used, direct acid–base titration is done with a strong acid of known concentration. HCl or H2SO4 can be used. Indirect back titration is used instead if strong acids were used to make the standard acid solution: strong base of known concentration (like NaOH) is used to neutralize the solution. In this case, the amount of ammonia is calculated as the difference between the amount of HCl and NaOH. In the case of direct titration, it is not necessary to know the exact amount of weak acid (e.g. boric acid) because it does not interfere with the titration (it does have to be in excess of ammonia to efficiently trap it). Thus, one standard solution is needed (e.g. HCl) in the direct titration, while two are needed (e.g. HCl and NaOH) in the back-titration. One of the suitable indicators for these titration reactions is Tashiro's indicator.[3]
In practice, this analysis is largely automated; specific catalysts accelerate the decomposition. Originally, the catalyst of choice was mercuric oxide. However, while it was very effective, health concerns resulted in it being replaced by cupric sulfate. Cupric sulfate was not as efficient as mercuric oxide, and yielded lower protein results. It was soon supplemented with titanium dioxide, which is currently the approved catalyst in all of the methods of analysis for protein in the Official Methods and Recommended Practices of AOAC International.[5]
Applications
The Kjeldahl method's universality, precision and reproducibility have made it the internationally recognized method for estimating the protein content in foods and it is the standard method against which all other methods are judged. It is also used to assay soils, waste waters, fertilizers and other materials. It does not, however, give a measure of true protein content, as it measures nonprotein nitrogen in addition to the nitrogen in proteins. This is evidenced by the 2007 pet food incident and the 2008 Chinese milk powder scandal, when melamine, a nitrogen-rich chemical, was added to raw materials to fake high protein contents. Also, different correction factors are needed for different proteins to account for different amino acid sequences. Additional disadvantages, such as the need to use concentrated sulfuric acid at high temperature and the relatively long testing time (an hour or more), compare unfavorably with the Dumas method for measuring crude protein content.[6]
Total Kjeldahl nitrogen
Total Kjeldahl nitrogen or TKN is the sum of nitrogen bound in organic substances, nitrogen in ammonia (NH3-N) and in ammonium (NH4+-N) in the chemical analysis of soil, water, or waste water (e.g. sewage treatment plant effluent).
Today, TKN is a required parameter for regulatory reporting at many treatment plants, and as a means of monitoring plant operations.
Conversion factors
TKN is often used as a surrogate for protein in food samples. The conversion from TKN to protein depends on the type of protein present in the sample and what fraction of the protein is composed of nitrogenous amino acids, like arginine and histidine. However, the range of conversion factors is relatively narrow. Example conversion factors, known as N factors, for foods range from 6.38 for dairy and 6.25 for meat, eggs, maize (corn) and sorghum to 5.83 for most grains; 5.95 for rice, 5.70 for wheat flour, and 5.46 for peanuts.[7] In practice, 6.25 is used for almost all food and feed regardless of applicability. The factor 6.25 is specifically required by US Nutrition Label regulations in the absence of another published factor. [8]
| Animal origin | Factor | Grass seeds | Factor | Beans and peanuts | Factor |
|---|---|---|---|---|---|
| Eggs | 6.25 | Barley | 5.83 | Castor bean | 5.30 |
| Meat | 6.25 | Corn (maize) | 6.25 | Jack bean | 6.25 |
| Milk | 6.38 | Millets | 5.83 | Lima bean | 6.25 |
| Oats | 5.83 | Navy bean | 6.25 | ||
| Rice | 5.95 | Mung bean | 6.25 | ||
| Rye | 5.83 | Soybean | 5.71 | ||
| Sorghum | 6.25 | Velvet bean | 6.25 | ||
| Wheat: Whole kernel | 5.83 | Peanuts | 5.46 | ||
| Wheat: Bran | 6.31 | ||||
| Wheat: Endosperm | 5.70 |
Sensitivity
The Kjeldahl method is poorly sensitive in the original version. Other detection methods have been used to quantify NH4+ after mineralisation and distillation, achieving improved sensitivity: in-line generator of hydride coupled to a plasma atomic emission spectrometer (ICP-AES-HG, 10–25 mg/L),[10] potentiometric titration (>0.1 mg of nitrogen), zone capillary electrophoresis (1.5 µg/ml of nitrogen),[11] and ion chromatography (0.5 µg/ml).[12]
Limitations
Kjeldahl method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in rings (e.g. pyridine, quinoline, isoquinoline) as nitrogen of these compounds does not convert to ammonium sulfate under the conditions of this method.
See also
- Dumas method, another nitrogen analysis method
- Devarda's alloy, a powerful reducing agent for nitrate analysis
- Bicinchoninic acid assay, a colorimetric assay for protein-nitrogen
- Combustion analysis another carbon, hydrogen and nitrogen analysis method
References
- ↑ Kjeldahl, J. (1883) "Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern" (New method for the determination of nitrogen in organic substances), Zeitschrift für analytische Chemie, 22 (1) : 366-383.
- ↑ Julius B. Cohen Practical Organic Chemistry 1910 Link to online text
- ↑ 3.0 3.1 3.2 3.3 3.4 Michałowski, T; Asuero, AG; Wybraniec, S (2013-02-12). "The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?". Journal of Chemical Education 90 (2): 191–197. doi:10.1021/ed200863p. ISSN 0021-9584. Bibcode: 2013JChEd..90..191M.
- ↑ "International Starch: ISI 24 Determination of Protein by Kjeldahl.". http://www.starch.dk/isi/methods/24prot.htm.
- ↑ AOAC International
- ↑ D. Julian McClements. "Analysis of Proteins". University of Massachusetts Amherst. http://www-unix.oit.umass.edu/~mcclemen/581Proteins.html.
- ↑ "CHAPTER 2: METHODS OF FOOD ANALYSIS". http://www.fao.org/docrep/006/y5022e/y5022e03.htm.
- ↑ "21 CFR 101.9 (c)(7)". https://www.ecfr.gov/cgi-bin/text-idx?SID=f676cd28ca7c11040bbd1de5d82f4e3b&mc=true&node=se21.2.101_19&rgn=div8.
- ↑ "Chapter 2: methods of food analysis". 2020-11-14. http://www.fao.org/3/y5022e/y5022e03.htm.
- ↑ A.M.Y. Jaber; N.A. Mehanna; S.M. Sultan (2009). "Determination of ammonium and organic bound nitrogen by inductively coupled plasma emission spectroscopy". Talanta 78 (4–5): 1298–1302. doi:10.1016/j.talanta.2009.01.060. PMID 19362191. http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.elsevier-693f1549-6e43-3e3e-bd8b-8d5b15d15276.
- ↑ "Intérêt de l'ECZ pour le dosage de l'azote total (méthode de Kjeldahl) - Blog Pharma Physic". 5 April 2012. http://blog.pharmaphysic.fr/ecz-dosage-azote-kjeldahl/#more-592.
- ↑ "Peut-on éviter l'étape de distillation dans la méthode Kjeldahl ? - Blog Pharma Physic". 26 April 2012. http://blog.pharmaphysic.fr/eviter-distillation-methode-kjeldahl/#more-83.
Bibliography
- Wastewater Engineering: Treatment and Reuse, Metcalf & Eddy, McGraw-Hill Higher Education; 4th edition, 1 May 2002, ISBN:978-0071241403
External links
 |

