Chemistry:Transition metal nitroso complexes
Transition metal nitroso complexes are coordination complexes containing one or more organonitroso ligands (RNO).[1]
Structure and bonding
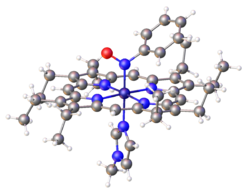
- thumb|right|Structure of the dye Naphthol Green B, which features of nitroso ligand bound to Fe(III).
Organic nitroso compounds bind to metals in several ways, but most commonly as monodentate N-bonded ligands. Also known are O-bonded, η2-N,O-bonded. Dimers of organic nitroso compounds also bind in a κ2--O,O bidentate manner.
Synthesis
Organic nitroso complexes can be prepared from preformed organic nitroso precursors. These precursors usually exist as N-N bonded dimers, but the dimer dissociates readily. This direct method is used to give W(CO)5(tert-BuNO) (where tert-Bu is (CH
3)
3C).[2] The Fe-porphyrin complex depicted below is prepared by this route. More complicated but more biorelevant routes involve degradation of precursors such as nitrobenzene and phenylhydroxylamine.[3]
- Ni(PEt
3)
4 + i–PrNO
2 → Ni(PEt
3)
2(η2−i–PrNO) + PEt
3 + OPEt
3 (Et = C2H5, i-Pr = (CH3)2CH)
The coupling of organic ligands and nitric oxide is yet another route.[1]
Connection to methemoglobinemia
Methemoglobinemia is a disorder where a large fraction of hemoglobin in one's blood has converted to inactive forms, generically called methemoglobin. Since methemoglobin is not an oxygen-carrier, methemoglobinemia is a serious disorder, sometimes fatal. Exposure to nitrobenzene, aniline, and their derivatives cause this disorder, which is attributed to their conversion to nitrosobenzene (and derivatives), which inactivate hemoglobin by forming a complex with the Fe center, precluding binding of O2.[4]
References
- ↑ Jump up to: 1.0 1.1 Lee, Jonghyuk; Chen, Li; West, Ann H.; Richter-Addo, George B. (2002). "Interactions of Organic Nitroso Compounds with Metals". Chemical Reviews 102 (4): 1019–1066. doi:10.1021/cr0000731. PMID 11942786.
- ↑ Pilato, R. S.; McGettigan, C.; Geoffroy, G. L.; Rheingold, A. L.; Geib, S. J. (1990). "tert-Butylnitroso Complexes. Structural Characterization of W(CO)5(N(O)Bu-tert) and [CpFe(CO)(PPh3)(N(O)Bu-tert)]+". Organometallics 9 (2): 312–17. doi:10.1021/om00116a004.
- ↑ Berman, R. S.; Kochi, J. K. (1980). "Kinetics and Mechanism of Oxygen Atom Transfer from Nitro Compounds Mediated by Nickel(0) Complexes". Inorganic Chemistry 19: 248–254. doi:10.1021/ic50203a050.
- ↑ Godbout, Nathalie; Sanders, Lori K.; Salzmann, Renzo; Havlin, Robert H.; Wojdelski, Mark; Oldfield, Eric (1999). "Solid-State NMR, Mössbauer, Crystallographic, and Density Functional Theory Investigation of Fe−O2and Fe−O2Analogue Metalloporphyrins and Metalloproteins". Journal of the American Chemical Society 121 (16): 3829–3844. doi:10.1021/ja9832820.
 |