Chemistry:Transition metal thiosulfate complex
A transition metal thiosulfate complex is a coordination complex containing one or more thiosulfate ligands. Thiosulfate occurs in nature and is used industrially, so its interactions with metal ions are of some practical interest.[1][2] Three binding modes are common: monodentate (κ1-), O,S-bidentate (κ2-),and bridging (μ-). Typically, thiosulfate complexes are prepared from thiosulfate salts. In some cases, they arise by oxidation of polysulfido complexes, or by binding of sulfur trioxide to sulfido ligands.[3]
Applications
Silver-thiosulfate complexes are produced by common photographic fixers. By dissolving silver halides the fixer stabilises the image. The dissolution process entails reactions involving the formation of 1:2 and 1:3 complexes (X = halide):[4] Fixation involves these chemical reactions (X = halide, typically Br−
):[5]
- AgX + 2 S
2O2−
3 → [Ag(S
2O
3)
2]3− + X− - AgX + 3 S
2O2−
3 → [Ag(S
2O
3)
3]5− + X−
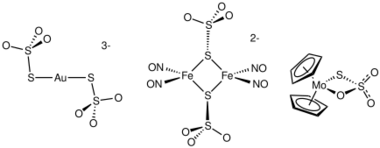
Sodium aurothiosulfate dihydrate, Na
3[Au(S
2O
3)
2] · 2H2O, has been widely discussed in the context of the extraction of gold from its ores. Presently cyanide salts are used on a large scale for this purpose with obvious risks.[6]
Naming
In the IUPAC Red Book the following terms may be used for thiosulfate as a ligand: trioxido-1κ3O-disulfato(S—S)(2−); trioxidosulfidosulfato(2−); thiosulfato; sulfurothioato. In the naming for thiosulfate salts, the final "o" is replaced by "e".[7] Thus, sodium aurothiosulfate could be called trisodium di(thiosulfato)aurate(I).
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 715. ISBN 978-0-08-037941-8.
- ↑ Trachevskii, V. V.; Zimina, S. V.; Rodina, E. P. (2008). "Thiosulfate Metal Complexes". Russian Journal of Coordination Chemistry 34 (9): 664–669. doi:10.1134/S1070328408090066.
- ↑ Kubas, G. J.; Ryan, R. R. (1984). "Reduction of Sulfur Dioxide by Cp2MH2 (M = Mo, W) to Cp2M(S2O3) and water. Molecular Structure and Reaction with Acids of an Organometallic Molybdenum-Thiosulfate Complex". Inorganic Chemistry 23 (20): 3181–3183. doi:10.1021/ic00188a030.
- ↑ Sowerby, A.L.M., ed (1961). Dictionary of Photography: A Reference Book for Amateur and Professional Photographers. London: Illife Books Ltd.. pp. 324–326.
- ↑ Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_001
- ↑ "Thiosulfate Leaching of Gold - a Review". Minerals Engineering 14 (2): 135–174. 2001. doi:10.1016/s0892-6875(00)00172-2.
- ↑ IUPAC Red Book p 329
 |