Chemistry:Sodium aurothiosulfate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intramuscular injection |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | AuNa3O6S4 |
| Molar mass | 490.17 g·mol−1 |
| (verify) | |
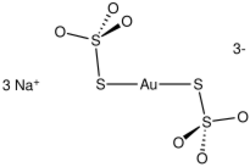
Sodium aurothiosulfate, or sanocrysin, is the inorganic compound with the formula Na
3[Au(S
2O
3)
2] · 2H2O. It is the trisodium salt of the coordination complex of gold(I), [Au(S
2O
3)
2]3−. The dihydrate, which is colorless, crystallizes with two waters of crystallization. The compound has some medicinal properties as well as potential for hydrometallurgy.
Structure
thumb|left|X-ray crystallographic structure of Na
3[Au(S
2O
3)
2] · 2H2O. Color code: red = O, orange = Au, yellow = S, violet = Na. Hydrogen atoms are omitted.
The anionic complex features a linear AuS2 core and is overall centrosymmetric. Like most other thiosulfate complexes, only the planetary sulfur of thiosulfate is coordinated to the metal.[1][2][3]
History
The salt is typically prepared by reduction of gold(III) chloride with thiosulfate:[3]
- 4 Na
2S
2O
3 + AuCl
3 → Na
3[Au(S
2O
3)
2] + Na
2S
4O
6 + 3 NaCl
The compound was first synthesized in 1845 by Mathurin-Joseph Fordos and A. Gélis who were researching chemicals used in the Daguerrotype photographic process. It then came to be called Fordos and Gélis salt.[4][5] It went out of interest until 1924 when it was noted as a chemotherapeutic agent for tuberculosis by Holger Møllgaard in Copenhagen.[6] Other methods of synthesis were then identified.[7]
Potential applications
Like several other gold compounds, this species is used as an antirheumatic.[8] The first placebo-controlled trial was probably conducted in 1931, when sanocrysin was compared with distilled water for the treatment of tuberculosis.[9]
Aurothiosulfate complexes have also been discussed in the context of the extraction of gold from its ores. The general approach would employ sodium or ammonium thiosulfate in place of cyanide salts as lixiviants.[10][11]
References
- ↑ "Crystal and molecular structure of trisodium dithiosulphate aurate(I), dihydrate, Na3[Au(S2O3)2],2H2O". Journal of Inorganic and Nuclear Chemistry 35 (9): 3191–3200. 1973. doi:10.1016/0022-1902(73)80019-3.
- ↑ "Vibrational spectrum of Sanocrysin". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 53 (2): 183–188. 1997. doi:10.1016/S1386-1425(97)83024-7. Bibcode: 1997AcSpA..53..183T.
- ↑ 3.0 3.1 Ruben, Helena.; Zalkin, Allan.; Faltens, Marjorie O.; Templeton, David H. (1974). "Crystal structure of sodium gold(I) thiosulfate dihydrate, Na3Au(S2O3)2.2H2O". Inorganic Chemistry 13 (8): 1836–1839. doi:10.1021/ic50138a011.
- ↑ "Recherches sur la liqueur d'or employée en photographie". C.-R. Acad. Sc. (Paris) 17: 629–630. 1843.
- ↑ "Action du perchlorure d'or sur l'hyposulfite de soude". Ann. Chim. Phys.. 3 13: 394–404. 1845.
- ↑ "Current Topics and Events" (in en). Nature 115 (2886): 272–277. 1925. doi:10.1038/115272c0. ISSN 0028-0836. Bibcode: 1925Natur.115S.272..
- ↑ "New methods for the preparation of sodium aurothiosulfate" (in en). Journal of the American Chemical Society 48 (1): 136–139. 1926. doi:10.1021/ja01412a018. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01412a018.
- ↑ "Gold-based therapeutic agents". Chemical Reviews 99 (9): 2589–2600. September 1999. doi:10.1021/cr980431o. PMID 11749494.
- ↑ Ethical and regulatory aspects of clinical research: readings and commentary. Johns Hopkins University Press. 2003. p. 140. ISBN 978-0801878138.
- ↑ "Thiosulfate Leaching of Gold - a Review". Minerals Engineering 14 (2): 135–174. 2001. doi:10.1016/s0892-6875(00)00172-2.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
 |
