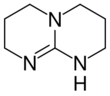
Chemistry:Triazabicyclodecene
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,4,6,7,8-Hexahydro-2H-pyrimido[1,2-a]pyrimidine | |||
| Other names
1,5,7-Triazabicyclo[4.4.0]dec-5-ene
TBD Hexahydropyrimidopyrimidine hpp | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H13N3 | |||
| Molar mass | 139.20 g/mol | ||
| Melting point | 125 to 130 °C (257 to 266 °F; 398 to 403 K) | ||
| Acidity (pKa) | 15.2 ± 1.0[2] (pKa of conjugate acid in water); 26.03[3] (pKa of conjugate acid in acetonitrile) | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H314 | |||
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Triazabicyclodecene (1,5,7-triazabicyclo[4.4.0]dec-5-ene or TBD) is an organic compound consisting of a bicyclic guanidine. For a charge-neutral compound, it is a relatively strong base that is effective for a variety of organic transformations. TBD is colorless solid that is soluble in a variety of solvents.[4]
Reactivity
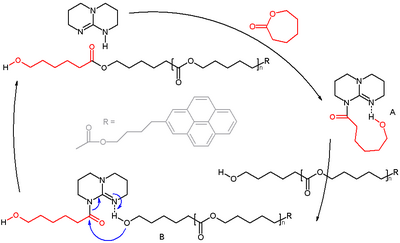
Mechanism proposed for the ring-opening polymerization of caprolactone to polycaprolactone by TBD.[5][6]
As a strong base, TBD fully deprotonates most phenols, carboxylic acids, and some C-acids.[7] It catalyzes a variety of reactions including Michael reactions, Henry reactions (nitroaldol reactions), transesterification reactions, and Knoevenagel condensations.[8]
Deprotonation at the 7-position gives a particularly electron-rich ligand as manifested in the redox properties of ditungsten tetra(hpp).
See also
- 1,8-Diazabicyclo(5.4.0)undec-7-ene, a structurally related strong base
- 7-Methyl-TBD, a methyl derivative of TBD
References
- ↑ 1,5,7-Triazabicyclo[4.4.0]dec-5-ene at Sigma-Aldrich
- ↑ Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta 87 (4): 385–395. doi:10.5562/cca2472. http://hrcak.srce.hr/file/194352.
- ↑ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ↑ Huczynski, Adam; Brzezinski, Bogumil (2008). "1,5,7-Triazabicyclo[4.4.0]dec-5-ene". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rn00786. ISBN 978-0471936237.
- ↑ Pratt, Russell C.; Lohmeijer, Bas G. G.; Long, David A.; Waymouth, Robert M.; Hedrick, James L. (2006). "Triazabicyclodecene: A Simple Bifunctional Organocatalyst for Acyl Transfer and Ring-Opening Polymerization of Cyclic Esters". J. Am. Chem. Soc. 128 (14): 4556–4557. doi:10.1021/ja060662+. PMID 16594676.
- ↑ Reaction specs: initiator 4-pyrenebutanol (pyrene enables end-group determination by UV–vis) and monomer caprolactone added in ratio 1:100, targeted degree of polymerization = 100, with TBD cat. 0.5% in benzene; 72% conversion in 8 hours; polydispersity index 1.16
- ↑ Huczyński, A.; Binkowska, I.; Jarczewski, A.; Brzezinski, B. (2007). "Spectroscopic studies of the 1:1 complexes of 4-nitrophenyl(bis(ethylsulfonyl))methane and phenyl(bis(ethylsulfonyl))methane with 7-methyl-1,5,7-triazabicyclo(4.4.0)dec-5-ene and 1,5,7-triazabicyclo(4.4.0)dec-5-ene". J. Mol. Struct. 841 (1–3): 133–136. doi:10.1016/j.molstruc.2007.01.005.
- ↑ Sabot, Cyrille; Kumar, Kanduluru Ananda; Meunier, Stéphane; Mioskowski, Charles (2007). "A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) under solvent-free conditions". Tetrahedron Lett. 48 (22): 3863–3866. doi:10.1016/j.tetlet.2007.03.146.
 |