Chemistry:Triazenes
thumb|200px|[[Dacarbazine is a triazene used in the treatment of melanoma and Hodgkin's lymphoma.[1]]] Triazenes are organic compounds that contain the functional group R1−NN=N−NR2R3, where the R are each any of various types of substituent groups.[2] Some anti-cancer medications and dyes are triazenes.[3] Formally, the triazenes are related to the unstable chemical triazene, H2N−N=NH.
Production
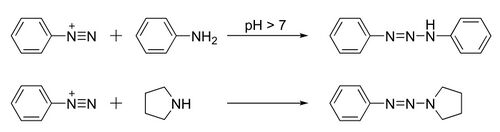
Triazenes are prepared from the N-coupling reaction between diazonium salts and primary or secondary amines.[4] The coupling reactions are typically mild, using a base such as sodium acetate,[5] sodium carbonate,[5] or sodium bicarbonate.[4]
The diazonium reagents are themselves available starting from amines. For symmetrical triazenes derived from primary amines, partial diazotization gives a mixture of the original amine and its diazo derivative that then couple with each other. For example, 1,3-diphenyltriazene (PhN=N−NHPh) can be made from aniline in a one-pot reaction.[5][6] For asymmetrical triazenes, for example (phenyldiazenyl)pyrrolidine (PhN=N−NC4H8), the diazonium salt must be pre-made.
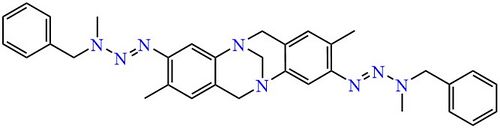
Analogues of Tröger's base containing a symmetric pair of asymmetric triazene side-chains have been obtained similarly.[7]
Reactions and applications
Triazenes derived from primary amines engage in tautomerism. In the case of symmetric triazenes, the tautomers are identical.
Triazenes can be converted to diazonium salts.[8]
File:Triazene 1.tif Triazenes decompose in the presence of protonating or alkylating agents into quaternary amines and diazonium salts; as such triazenes have been used as an in situ source of diazonium that reacted with sodium sulfide to give the corresponding thiophenols.[5] A strategy for the protection and deprotection of sensitive secondary amines is based on this principle.[9]
Polymeric triazenes are applied as conductive and absorbent materials.[10] Triazenes have been used in the synthesis of cinnoline, functionalized lactams, and coumarins.[9][8]
References
- ↑ "Dacarbazine". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/dacarbazine.html.
- ↑ Suleymanov, Abdusalom A.; Severin, Kay (2021-03-22). "Vinyl and Alkynyl Triazenes: Synthesis, Reactivity, and Applications" (in en). Angewandte Chemie International Edition 60 (13): 6879–6889. doi:10.1002/anie.202011031. ISSN 1433-7851. https://onlinelibrary.wiley.com/doi/10.1002/anie.202011031.
- ↑ Berneth, Horst (2008). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_487.pub2.
- ↑ 4.0 4.1 Sengupta, Saumitra; Sadhukhan, Subir K. (2002). "Synthesis of Symmetrical trans-Stilbenes by a Double Heck Reaction of (Arylazo)amines with Vinyltriethoxysilane: trans-4,4′-Dibromostilbene". Organic Syntheses 79: 52. doi:10.15227/orgsyn.079.0052.
- ↑ 5.0 5.1 5.2 5.3 Kazem-Rostami, M.; Khazaei, A.; Moosavi-Zare, A. R.; Bayat, M.; Saednia, S. (2012). "Novel One-Pot Synthesis of Thiophenols from Related Triazenes under Mild Conditions". Synlett 23 (13): 1893–1896. doi:10.1055/s-0032-1316557.
- ↑ Hartman, W. W.; Dickey, J. B. (1934). "Diazoaminobenzene". Organic Syntheses 14: 24. doi:10.15227/orgsyn.014.0024.
- ↑ Kazemostami, Masoud (2017). "Facile preparation of Ʌ-shaped building blocks: Hünlich-base derivatization". Synlett 28: 1641–1645. doi:10.1055/s-0036-1588180.
- ↑ 8.0 8.1 Kimball, D. B.; Haley, M. M. (2002). "Triazenes: A Versatile Tool in Organic Synthesis". Angewandte Chemie International Edition 41 (18): 3338–51. doi:10.1002/1521-3773(20020916)41:18<3338::AID-ANIE3338>3.0.CO;2-7. PMID 12298030.
- ↑ 9.0 9.1 Lazny, R.; Poplawski, J.; Köbberling, J.; Enders, D.; Bräse, S. (1999). "Triazenes: A Useful Protecting Strategy for Sensitive Secondary Amines". Synlett 1999 (8): 1304–1306. doi:10.1055/s-1999-2803.
- ↑ Khazaei, A.; Zare, A.; Moosavi-Zare, A. R.; Sadeghpour, M.; Afkhami, A. (2013). "Synthesis, characterization, and application of a triazene-based polysulfone as a dye adsorbent". Journal of Applied Polymer Science 129 (6): 3439–3446. doi:10.1002/app.39069.
 |