Chemistry:Triflidic acid
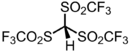
| |
| Names | |
|---|---|
| Preferred IUPAC name
[Bis(trifluoromethanesulfonyl)methanesulfonyl]tri(fluoro)methane | |
| Other names
Triflidic acid, tris(triflyl)methane, tris[(trifluoromethyl)sulfonyl]methane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4F9S3O6H | |
| Molar mass | 412.23 g/mol |
| Appearance | Colorless solid |
| Melting point | 69.2 °C (156.6 °F; 342.3 K) |
| Miscible | |
| Acidity (pKa) | –18.6 (aqueous, est.)[1] |
| Hazards | |
| Main hazards | Corrosive, eye irritant |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H314, H335 | |
| P260, P261, P264, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triflidic acid (IUPAC name: tris[(trifluoromethyl)sulfonyl]methane, abbreviated formula: Tf3CH) is an organic superacid. It is one of the strongest known carbon acids and is among the strongest Brønsted acids in general, with an acidity exceeded only by the carborane acids. Notably, triflidic acid is estimated to have an acidity 104 times that of triflic acid (pKaaq ~ –14), as measured by its acid dissociation constant. It was first prepared in 1987 by Seppelt and Turowsky by the following route:[2]
(1) Tf2CH2 + 2CH3MgBr → Tf2C(MgBr)2 + 2CH4
(2) Tf2C(MgBr)2 + TfF → Tf3C(MgBr) + MgBrF
(3) Tf3C(MgBr) + H2SO4 → Tf3CH + MgBrHSO4
In its anionic form, the lanthanide salts of triflidic acid ("triflides") have been shown to be more efficient Lewis acids than the corresponding triflates.[3][4] The triflide anion has also been employed as the anionic component of ionic liquids.[5]
See also
References
- ↑ Barrett, A. G. M.; Braddock, D. C.; Raju, G. S. (2004). "Tris[(trifluoromethyl)sulfonyl]methane and Related Salts". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00441. ISBN 9780470842898.
- ↑ Turowsky, Lutz; Seppelt, Konrad (1988-06-01). "Tris[(trifluoromethyl)sulfonyl]methane, HC(SO2CF3)3". Inorganic Chemistry 27 (12): 2135–2137. doi:10.1021/ic00285a025. ISSN 0020-1669.
- ↑ Waller, Francis J.; Barrett, Anthony G. M.; Braddock, D. Christopher; Ramprasad, Dorai; McKinnell, R. Murray; White, Andrew J. P.; Williams, David J.; Ducray, Richard (1999-04-01). "Tris(trifluoromethanesulfonyl)methide ("Triflide") Anion: Convenient Preparation, X-ray Crystal Structures, and Exceptional Catalytic Activity as a Counterion with Ytterbium(III) and Scandium(III)". The Journal of Organic Chemistry 64 (8): 2910–2913. doi:10.1021/jo9800917. ISSN 0022-3263. PMID 11674365.
- ↑ Ishihara, Kazuaki; Hiraiwa, Yukihiro; Yamamoto, Hisashi (2000-01-01). "Homogeneous Debenzylation Using Extremely Active Catalysts: Tris(triflyl)methane, Scandium(III) Tris(triflyl)methide, and Copper(II) Tris(triflyl)methide" (in en). Synlett 2000 (1): 80–82. doi:10.1055/s-2000-6436. ISSN 0936-5214.
- ↑ Johansson, Katarina M.; Adebahr, Josefina; Howlett, Patrick C.; Forsyth, Maria; MacFarlane, Douglas R. (2007-01-01). "N-Methyl-N-Alkylpyrrolidinium Bis(perfluoroethylsulfonyl)amide ([NPf2]) and Tris(trifluoromethanesulfonyl)methide ([CTf3]) Salts: Synthesis and Characterization". Australian Journal of Chemistry 60 (1): 57–63. doi:10.1071/ch06299.
 |

