Chemistry:Trinitroethylorthocarbonate
From HandWiki
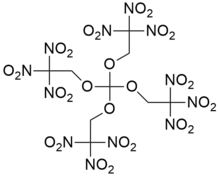
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1-Trinitro-2-[tris(2,2,2-trinitroethoxy)methoxy]ethane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C9H8N12O28 | |
| Molar mass | 732.219 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 161 °C (322 °F; 434 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trinitroethylorthocarbonate also known as TNEOC is an oxidizer with excellent chemical stability.[citation needed] Its explosion point is 238 °C, and it begins to be decomposed at 200 °C. Its explosion heat is 5.797 J/g and specific volume is 694 L/kg.[1] Its structure is closely related to that of trinitroethylorthoformate (TNEOF). Both are highly explosive and very shock-sensitive, and may be dissolved in nitroalkanes to reduce their shock-sensitivity.[1]
Synthesis
Trinitroethanol reacts with carbon tetrachloride under a catalyst of FeCl3.
- [math]\ce{ \underset{Carbon\ tetrachloride}{CCl4} + \underset{Trinitroethanol}{4HOCH2C(NO2)3} ->[\ce{FeCl3}] {TNEOC} + 4HCl }[/math]
References
- ↑ 1.0 1.1 Liu, Jiping (2015). Liquid Explosives. Springer. pp. 5, 6, 8, 136, 309. ISBN 9783662458471. https://books.google.com/books?id=NGYiBgAAQBAJ&pg=PA8. Retrieved 26 March 2016.
 |

