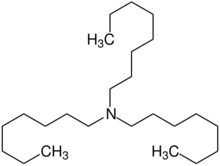
Chemistry:Trioctylamine
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Di(octyl)octan-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H51N | |
| Molar mass | 353.679 g·mol−1 |
| Density | 0.81 g/cm3[1] |
| Melting point | −34.6 °C (−30.3 °F; 238.6 K) |
| 0.050 mg/l | |
| Viscosity | 7.862 mPa.s |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H315, H319, H335, H360, H372, H410, H411 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P302+352, P304+340, P305+351+338, P308+313, P312, P314, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trioctylamine is a clear and colorless chemical compound in the group of aliphatic amines and tertiary amines.
Physical and chemical properties
It is a clear colorless liquid and can be converted to the amine hydrochloride etherate which is recrystallized four times from diethyl ether at -30 °C. Neutralization of this salt regenerates the free amine which can be distilled under high vacuum. It has the melting point of −34 °C; boiling point of 164-168 °C at 0.7 mmHg and 365-367 °C at 1 atm; density of 0.810 g/mL at 20 °C; refractive index of n20/D 1.449; flash point of >230 °F; storage temperature is below 30 °C. Trioctylamine is easily soluble in chloroform but insoluble in water. It is air sensitive.[2] There is a safety hazard for this chemical compound. It can cause skin irritation, serious eye irritation, and respiratory irritation. It can damage fertility or the unborn child and cause damage to organs through prolonged or repeated exposure. It is very toxic to aquatic life with long lasting effects.
Applications
Trioctylamine is can be used to extract monocarboxylic acids such as acetic acid, and also precious metals. A formulation containing metoxuron mixed with an emulsion containing trioctylamine 50%, atlox 4851 B 15%, and isopropanol 35% was active as a potato defoliant. Trioctylamine can be used to extract monocarboxylic acid for equilibria and correlation of apparent reactive equilibrium constant.[3] Liquid-liquid equilibria for aqueous solutions of carboxylic acids with trioctylamine in various diluents were determined at various trioctylamine concentrations. The loading of trioctylamine for a given carboxylic acid depends on the nature of the solute and its concentration. The apparent extraction equilibrium constants depend on the hydrophobicity and acidity of the carboxylic acid, as well as the specific basicity of trioctylamine.[4] Trioctylamine production can be used as a mineral extraction reagent, an extractant for nuclear reprocessing, and its use as an extractant for identification of dyes may result in its release to the environment through various waste streams.
External links
- ↑ "Trioctylamine CAS#:1116-76-3". https://www.chemsrc.com/en/cas/1116-76-3_15351.html.
- ↑ "Trioctylamine T81000". https://www.sigmaaldrich.com/catalog/product/aldrich/t81000?lang=en®ion=US.
- ↑ Qin, Wei; Li, Zhenyu; Dai, Youyuan (November 2003). "Extraction of Monocarboxylic Acids with Trioctylamine: Equilibria and Correlation of Apparent Reactive Equilibrium Constant" (in en). Industrial & Engineering Chemistry Research 42 (24): 6196–6204. doi:10.1021/ie021049b. ISSN 0888-5885.
- ↑ "Nanostructured Materials Through Ultrasonic Spray Pyrolysis" (in en). Sigma-Aldrich. https://www.sigmaaldrich.com/technical-documents/articles/material-matters/ultrasonic-spray-pyrolysis.html.
 |

