Chemistry:Triphenylethanol
From HandWiki
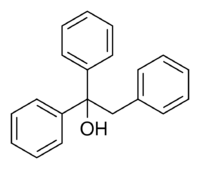
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,2-Triphenylethan-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H18O | |
| Molar mass | 274.363 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Triphenylethanol, or 1,1,2-triphenylethanol, is an organic compound with a condensed structural formula of (C6H5)2C(OH)CH2C6H5, and is related to triphenylethylene, from which it can be prepared by hydration. It is the structural analog of two drugs, the never-marketed antiestrogen ethamoxytriphetol (MER-25)[1]:7 and the withdrawn lipid-lowering agent triparanol,[2] as both contain the 1,1,2-triphenylethanol moiety within their structure.
There are three isomeric compounds with a "triphenylethanol" structure:
- 1,1,2-triphenylethanol;
- 1,2,2-triphenylethanol, with a condensed structural formula of (C6H5)2CHCH(C6H5)OH, which exists as a pair of enantiomers; and,
- 2,2,2-triphenylethanol, with a condensed structural formula of (C6H5)3CCH2OH.
References
- ↑ Jordan, V. Craig (2013). "A Century of Deciphering the Control Mechanisms of Estrogen Action in Breast Cancer: The Origins of Targeted Therapy and Chemoprevention". in Jordan, V. Craig. Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health: Progress and Promise. World Scientific. pp. 1-29. ISBN 978-1-84816-959-3. https://books.google.com/books?id=ZM26CgAAQBAJ&pg=PA7.
- ↑ Bulletin of the National Institute of Sciences of India. National Institute of Sciences of India. 1964. p. 5. https://books.google.com/books?id=Ktg6AAAAMAAJ.
 |

