Chemistry:Triphenyltin hydroxide
From HandWiki

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triphenylstannanol | |
| Identifiers | |
3D model (JSmol)
|
|
| 4139186 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 7194 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2786 2588 |
| |
| |
| Properties | |
| C18H16OSn | |
| Molar mass | 367.035 g·mol−1 |
| Hazards | |
| GHS pictograms |     
|
| GHS Signal word | Danger |
| H301, H311, H315, H318, H330, H335, H351, H361, H372, H410 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P284, P301+310, P302+352, P304+340, P305+351+338, P308+313, P310, P312, P314, P320, P321, P322, P330, P332+313, P361 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Triphenyltin hydroxide is an organotin compound with formula Sn(C6H5)3OH. Triphenyltin hydroxide is used as a fungicide for potatoes, sugar beets, and pecans. It was first registered for use as a pesticide in the United States in 1971.[1]
Structure
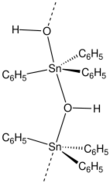
While triphenyltin hydroxide is often depicted as a monomer, it crystallizes as a polymer with a bridging hydroxide groups.[2] The Sn-O distances are 2.18 and 2.250 Å. Many organotin compounds engage in similar aggregation equilibria.
References
- ↑ "R.E.D. Facts: Triphenyltin Hydroxide". U.S. EPA. http://www.epa.gov/oppsrrd1/REDs/factsheets/0099fact.pdf.
- ↑ Christopher Glidewell; John N. Low; João A. S. Bomfim; Carlos A. L. Filgueiras; James L. Wardell (2002). "catena-Poly[[triphenyltin(IV)]-μ-hydroxo-κ2O:O] at 120 K". Acta Crystallographica Section C: Crystal Structure Communications 58 (Pt 4): M199-201. doi:10.1107/S0108270102001798. PMID 11932514.
 |
