Chemistry:Tropic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Hydroxy-2-phenylpropanoic acid | |
| Other names
2-Phenylhydracrylic acid; Tropate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | C011377 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
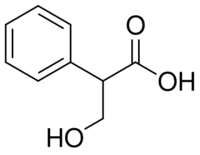
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
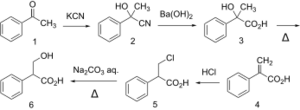
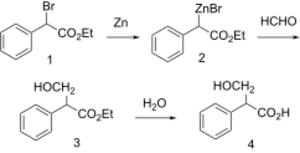
Tropic acid is a chemical with IUPAC name 3-hydroxy-2-phenylpropanoic acid and condensed structural formula HOCH2CHPhCOOH. It is a laboratory reagent used in the chemical synthesis of atropine and hyoscyamine. Tropic acid is a chiral substance, existing as either a racemic mixture or as a single enantiomer.
Synthesis
| Tropic acid synthesis 1:[1] | Tropic acid synthesis 2:[1] |
|---|---|
References
- ↑ 1.0 1.1 Gadzikowska, M; Grynkiewicz, G (2002). "Tropane alkaloical and phytochemical analysis". Acta Poloniae Pharmaceutica 59 (2): 149–60. PMID 12365608.
- ↑ Sletzinger, Paulsen, U.S. Patent 2,390,278 (1945 to Merck & Co.).
- ↑ Blicke, U.S. Patent 2,716,650 (1955 to University of Michigan).
- ↑ Blicke, Frederick Franklin, "Verfahren zur Herstellung von α-substituierten β-Oxypropionsäuren [Process for the preparation of α-substituted β-oxypropionic acids]", DE patent 923426, published 1955-02-14
- ↑ Blicke, F. F., Raffelson, H., Barna, B. (January 1952). "The Preparation of Tropic Acid". Journal of the American Chemical Society. 74 (1): 253–253. doi:10.1021/ja01121a504.
 |