Chemistry:URB602
From HandWiki
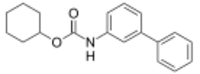
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexyl ([1,1′-biphenyl]-3-yl)carbamate | |
| Other names
[1,1′-Biphenyl]-3-yl-carbamic acid, cyclohexyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H21NO2 | |
| Molar mass | 295.382 g·mol−1 |
| Appearance | Crystalline solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
URB602 ([1,1'-biphenyl]-3-yl-carbamic acid, cyclohexyl ester) is a compound that has been found to inhibit hydrolysis of monoacyl glycerol compounds, such as 2-arachidonoylglycerol (2-AG) and 2-oleoylglycerol (2-OG). It was first described in 2003.[1] A study performed in 2005 found that the compound had specificity for metabolizing 2-AG over anandamide (another cannabinoid ligand) in rat brain presumably by inhibiting the enzyme monoacylglycerol lipase (MAGL), which is the primary metabolic enzyme of 2-AG.[2] However, subsequent studies have shown that URB602 lacks specificity for MAGL inhibition in vitro.[3]
References
- ↑ Tarzia, G; Duranti, A; Tontini, A; Piersanti, G; Mor, M; Rivara, S; Plazzi, PV; Park, C et al. (2003). "Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors". Journal of Medicinal Chemistry 46 (12): 2352–60. doi:10.1021/jm021119g. PMID 12773040. http://www.escholarship.org/uc/item/7sb397f0.
- ↑ Hohmann, Andrea G.; Suplita, Richard L.; Bolton, Nathan M.; Neely, Mark H.; Fegley, Darren; Mangieri, Regina; Krey, Jocelyn F.; Michael Walker, J. et al. (2005). "An endocannabinoid mechanism for stress-induced analgesia". Nature 435 (7045): 1108–12. doi:10.1038/nature03658. PMID 15973410. Bibcode: 2005Natur.435.1108H. https://cloudfront.escholarship.org/dist/prd/content/qt4gn3454w/qt4gn3454w.pdf.
- ↑ Vandevoorde, S; Jonsson, K-O; Labar, G; Persson, E; Lambert, D M; Fowler, C J (2007). "Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysisin vitro". British Journal of Pharmacology 150 (2): 186–91. doi:10.1038/sj.bjp.0706971. PMID 17143303.
 |
