Biology:Monoacylglycerol lipase
| acylglycerol lipase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
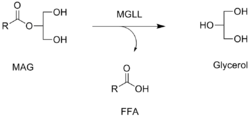 Reaction catalyzed by MGLL, in which a free fatty acid (FFA) is released from a monoacylglycerol (MAG) | |||||||||
| Identifiers | |||||||||
| EC number | 3.1.1.23 | ||||||||
| CAS number | 9040-75-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| monoglyceride lipase | |
|---|---|
| Identifiers | |
| Symbol | MGLL |
| NCBI gene | 11343 |
| HGNC | 17038 |
| OMIM | 609699 |
| RefSeq | NM_007283 |
| UniProt | Q99685 |
| Other data | |
| EC number | 3.1.1.23 |
| Locus | Chr. 3 p13-q13.33 |
Monoacylglycerol lipase (EC 3.1.1.23; systematic name glycerol-ester acylhydrolase, also known as MAG lipase, acylglycerol lipase, MAGL, MGL or MGLL) is an enzyme that, in humans, is encoded by the MGLL gene.[1][2][3] MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.[2][4]
Function
Monoacylglycerol lipase catalyzes a reaction that uses water molecules to break the glycerol monoesters of long-chain fatty acids:
- hydrolyses glycerol monoesters of long-chain fatty acids
It functions together with hormone-sensitive lipase (LIPE) to hydrolyze intracellular triglyceride stores in adipocytes and other cells to fatty acids and glycerol. MGLL may also complement lipoprotein lipase (LPL) in completing hydrolysis of monoglycerides resulting from degradation of lipoprotein triglycerides.[5]
Monoacylglycerol lipase is a key enzyme in the hydrolysis of the endocannabinoid 2-arachidonoylglycerol (2-AG).[6][7] It converts monoacylglycerols to the free fatty acid and glycerol. The contribution of MAGL to total brain 2-AG hydrolysis activity has been estimated to be ~85% (ABHD6 and ABHD12 are responsible for ~4% and ~9%, respectively, of the remainder),[8][9] and this in vitro estimate has been confirmed in vivo by the selective MAGL inhibitor JZL184.[10] Chronic inactivation of MAGL results in massive (>10-fold) elevations of brain 2-AG in mice, along with marked compensatory downregulation of CB1 receptors in selective brain areas.[11]
Inhibitors
MAGL enzyme inhibitors (URB602, URB754, JZL184) produce cannabinoid behavioral effects in mice.[10]
Further examples include:
- KML-29
- JZL195
- JNJ-42165279
- JW 642
See also
- Endocannabinoid enhancer
- Endocannabinoid reuptake inhibitor
- Triacylglycerol lipase
- Fatty acid amide hydrolase
References
- ↑ "A novel poxvirus gene and its human homolog are similar to an E. coli lysophospholipase". Virus Research 52 (2): 157–67. December 1997. doi:10.1016/S0168-1702(97)00122-6. PMID 9495531.
- ↑ 2.0 2.1 "cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases". The Journal of Biological Chemistry 272 (43): 27218–23. October 1997. doi:10.1074/jbc.272.43.27218. PMID 9341166.
- ↑ "Entrez Gene: monoglyceride lipase". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=11343.
- ↑ "Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue". The Journal of Biological Chemistry 251 (3): 813–9. February 1976. doi:10.1016/S0021-9258(17)33857-7. PMID 1249056.
- ↑ "Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene". Gene 272 (1–2): 11–8. July 2001. doi:10.1016/S0378-1119(01)00559-5. PMID 11470505.
- ↑ "Brain monoglyceride lipase participating in endocannabinoid inactivation". Proceedings of the National Academy of Sciences of the United States of America 99 (16): 10819–24. August 2002. doi:10.1073/pnas.152334899. PMID 12136125. Bibcode: 2002PNAS...9910819D.
- ↑ "Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus". Nature Neuroscience 8 (9): 1139–41. September 2005. doi:10.1038/nn1521. PMID 16116451. https://escholarship.org/uc/item/085499pd.
- ↑ Cannabinoid Receptors—Advances in Research and Application: 2012 Edition: ScholarlyBrief. ScholarlyEditions. 26 December 2012. pp. 68–. ISBN 978-1-4816-0672-1. https://books.google.com/books?id=QZqtIuUdJnQC&pg=PA68.
- ↑ "A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol". Chemistry & Biology 14 (12): 1347–56. December 2007. doi:10.1016/j.chembiol.2007.11.006. PMID 18096503.
- ↑ 10.0 10.1 "Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects". Nature Chemical Biology 5 (1): 37–44. January 2009. doi:10.1038/nchembio.129. PMID 19029917.
- ↑ "The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors". Acta Physiologica 204 (2): 267–76. February 2012. doi:10.1111/j.1748-1716.2011.02280.x. PMID 21418147.
- "Simultaneous purification and comparative characterization of six serine hydrolases from rat liver microsomes". Arch. Biochem. Biophys. 200 (2): 547–59. 1980. doi:10.1016/0003-9861(80)90386-0. PMID 6776896.
- "A study of a monoglyceride-hydrolyzing enzyme of intestinal mucosa". J. Biol. Chem. 241 (10): 2306–10. 1966. doi:10.1016/S0021-9258(18)96621-4. PMID 5916497.
External links
- Monoacylglycerol+lipases at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 3.1.1.23
- Monoglyceride lipase (MGLL) Human Protein Atlas
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |

