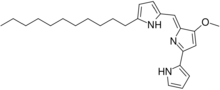
Chemistry:Undecylprodigiosin
| |
| Names | |
|---|---|
| IUPAC name
(2Z,5Z)-3-Methoxy-5-pyrrol-2-ylidene-2-[(5-undecyl-1H-pyrrol-2-yl)methylidene]pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H35N3O | |
| Molar mass | 393.575 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Undecylprodigiosin is an alkaloid produced by some Actinomycetes bacteria. It is a member of the prodiginines group of natural products and has been investigated for potential antimalarial activity.
Natural sources
Undecylprodigiosin is a secondary metabolite found in some Actinomycetes, for example Actinomadura madurae, Streptomyces coelicolor and Streptomyces longisporus.[1]
Production
Biosynthesis
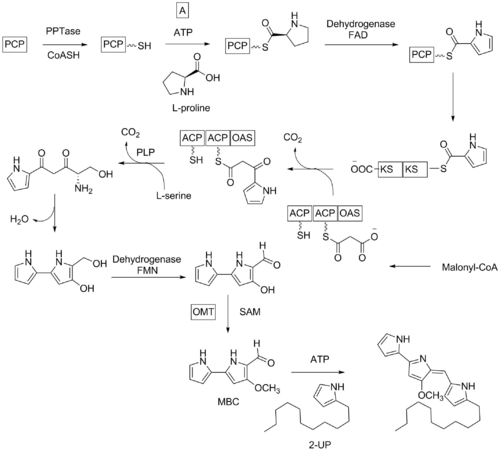
The biosynthesis of undecylprodigiosin starts with PCP apoprotein which is transformed into the holoprotein using acetyl CoA and PPtase then adenylation occurs utilizing L-proline and ATP. The resulting molecule is then oxidized by dehydrogenase enzyme. Elongation by decarboxylative condensation with malonyl CoA is followed by another decarboxylative condensation with L-serine using α-oxamine synthase (OAS) domain. The compound is then cyclized, oxidized with dehydrogenase and methylated with SAM to give 4-methoxy-2,2′-bipyrrole-5-carboxaldehyde (MBC) intermediate which react with 2-undecylpyrrole (2-UP) to give undecylprodigiosin.[2]
Laboratory
The first total synthesis of the undecylprodigiosin was published in 1966, confirming the chemical structure. As with the biosynthesis, the key intermediate was MBC.[2][3]
Uses
As with other prodiginines, the compound has been investigated for its pharmaceutical potential as anticancer, immunosuppressant, or antimalarial agent.[1][4]
References
- ↑ Jump up to: 1.0 1.1 "The biosynthesis and regulation of bacterial prodiginines". Nature Reviews Microbiology 4 (12): 887–899. 2006. doi:10.1038/nrmicro1531. PMID 17109029. https://www.researchgate.net/publication/6688845.
- ↑ Jump up to: 2.0 2.1 Hu, Dennis X.; Withall, David M.; Challis, Gregory L.; Thomson, Regan J. (17 June 2016). "Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products". Chemical Reviews 116 (14): 7818–7853. doi:10.1021/acs.chemrev.6b00024. PMID 27314508.
- ↑ Wasserman, H. H.; Rodgers, G. C.; Keith, D. D. (1966). "The structure and synthesis of undecylprodigiosin. A prodigiosin analogue from Streptomyces". Chemical Communications (22): 825–826. doi:10.1039/C19660000825.
- ↑ Stankovic, Nada; Senerovic, Lidija; Ilic-Tomic, Tatjana; Vasiljevic, Branka; Nikodinovic-Runic, Jasmina (2014). "Properties and applications of undecylprodigiosin and other bacterial prodigiosins". Applied Microbiology and Biotechnology 98 (9): 3841–3858. doi:10.1007/s00253-014-5590-1. PMID 24562326.
 |