Chemistry:Vanillic acid
|
| |||
| Names | |||
|---|---|---|---|
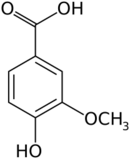
| Preferred IUPAC name
4-Hydroxy-3-methoxybenzoic acid | |||
| Other names
4-Hydroxy-m-anisic acid, Vanillate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.[2][3]
Occurrence in nature
The highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis,[4] an herb indigenous to China, which is used in traditional Chinese medicine.
Occurrences in food
Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in vanillic acid (1616±94 mg/kg).[5] It is one of the main natural phenols in argan oil.[citation needed] It is also found in wine and vinegar.[6]
Metabolism
Vanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions.[7]
Synthesis
Vanillic acid can be obtained from the oxidation of vanillin by various oxidizing agents. With Pd/C, NaBH4, and KOH as the oxidizing agent, the conversion was reported to occur in ~89% yield.[8]
References
- ↑ "Vanillic acid (4-hydroxy-3-methoxybenzoic acid)". chemicalland21.com. http://chemicalland21.com/lifescience/foco/VANILLIC%20ACID.htm.
- ↑ "A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus". J. Biotechnol. 50 (2–3): 107–113. October 1996. doi:10.1016/0168-1656(96)01552-0. PMID 8987621.
- ↑ "Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13". Appl. Environ. Microbiol. 66 (6): 2311–2317. June 2000. doi:10.1128/AEM.66.6.2311-2317.2000. PMID 10831404.
- ↑ Duke, JA (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, 999 edition. ISBN 978-0-8493-3865-6. http://www.ars-grin.gov/cgi-bin/duke/chemical.pl?VANILICACID. Retrieved 2012-01-07.
- ↑ "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". J Agric Food Chem 56 (12): 4631–4636. Jun 2008. doi:10.1021/jf800161u. PMID 18522407.
- ↑ Gálvez, Miguel Carrero; Barroso, Carmelo García; Pérez-Bustamante, Juan Antonio (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung 199: 29–31. doi:10.1007/BF01192948.
- ↑ Pietta, P. G.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazzoni, P.; Bombardelli, E. (1998). "Catechin metabolites after intake of green tea infusions". BioFactors 8 (1–2): 111–8. doi:10.1002/biof.5520080119. PMID 9699018.
- ↑ "Environmentally benign oxidation reaction of aldehydes to their corresponding carboxylic acids using Pd/C with NaBH4 and KOH". Tetrahedron Lett. 48 (22): 3835–3839. 2007. doi:10.1016/j.tetlet.2007.03.151.
 |