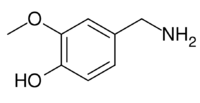
Chemistry:Vanillylamine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(Aminomethyl)-2-methoxyphenol | |
| Other names
4-Hydroxy-3-methoxybenzylamine
α-Amino-2-methoxy-p-cresol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H11NO2 | |
| Molar mass | 153.181 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Vanillylamine is a chemical compound that is an intermediate in the biosynthesis of capsaicin.[1] Vanillylamine is produced from vanillin by the enzyme vanillin aminotransferase.[2] It is then converted with 8-methyl-6-nonenoic acid into capsaicin by the enzyme capsaicin synthase.[2]
Reactions
Acylation of vanillylamine using Schotten-Baumann reactions can provide amide derivatives.[3] Examples include nonivamide (a component of some pepper sprays), olvanil, and arvanil.
References
- ↑ Edward Leete and Mary C. L. Louden (1968). "Biosynthesis of capsaicin and dihydrocapsaicin in Capsicum frutescens". J. Am. Chem. Soc. 90 (24): 6837–6841. doi:10.1021/ja01026a049. PMID 5687710.
- ↑ 2.0 2.1 "MetaCyc Pathway: capsaicin biosynthesis". MetaCyc. http://metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-5710.
- ↑ Wang, Bo; Yang, Fan; Shan, Yi-Fan; Qiu, Wen-Wei; Tang, Jie (2009). "Highly efficient synthesis of capsaicin analogues by condensation of vanillylamine and acyl chlorides in a biphase H2O/CHCl3 system". Tetrahedron 65 (27): 5409–5412. doi:10.1016/j.tet.2009.04.046. ISSN 00404020.
 |
