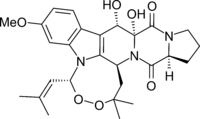
Chemistry:Verruculogen
| |
| Names | |
|---|---|
| Preferred IUPAC name
(5R,10S,10aR,14aS,15bS)-10,10a-Dihydroxy-7-methoxy-2,2-dimethyl-5-(2-methylprop-1-en-1-yl)-1,10,10a,12,13,14,14a,15b-octahydro-5H,15H-3,4-dioxa-5a,11a,15a-triazacycloocta[lm]indeno[5,6-b]fluorene-11,15(2H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| Properties | |
| C27H33N3O7 | |
| Molar mass | 511.575 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Verruculogen is a mycotoxin produced by certain strains of aspergillus that belongs to a class of naturally occurring 2,5-diketopiperazines.[1] It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a Salmonella/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.[2]
Synthesis
Both verruculogen and its isoprenyl derivative fumitremorgin A belong to the only family of alkaloids with an eight-membered endoperoxide ring, and both have been synthesised involving ligand-controlled C–H borylation.[3]
References
- ↑ Borthwick AD (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ↑ "Verruculogen from Penicillium verruculosum". http://www.sigmaaldrich.com/catalog/product/sigma/v7755?lang=en®ion=US.
- ↑ "Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C–H Borylation". Journal of the American Chemical Society 137 (32): 10160–10163. August 2015. doi:10.1021/jacs.5b07154. PMID 26256033.
 |

