Chemistry:Withaferin A
| |
| Names | |
|---|---|
| IUPAC name
(22R)-4β,27-Dihydroxy-5,6β:22,26-diepoxy-5β-ergosta-2,24-diene-1,26-dione
| |
| Systematic IUPAC name
(4S,4aR,5aR,6aS,6bS,9R,9aS,11aS,11bR)-4-Hydroxy-9-{(1S)-1-[(2R)-5-(hydroxymethyl)-4-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl]ethyl}-9a,11b-dimethyl-5a,6,6a,6b,7,8,9,9a,10,11,11a,11b-dodecahydrocyclopenta[1,2]phenanthro[8a,9-b]oxiren-1(4H)-one | |
| Other names
Withaferine A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H38O6 | |
| Molar mass | 470.606 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
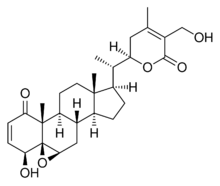
Withaferin A is a steroidal lactone, derived from Acnistus arborescens,[1] Withania somnifera[2] and other members of family Solanaceae. It is the first member of the withanolide class of ergostane type product to be discovered.
Structure
Withanolides are a group of naturally occurring C28- steroidal lactones. They contain four cycloalkane ring structures, three cyclohexane rings and one cyclopentane ring.[3] Withaferin A is highly reactive because of the ketone-containing unsaturated A ring, the epoxide in the B ring, and the unsaturated lactone ring. The double bond in ring A and the epoxide ring are mainly responsible for the cytotoxicity. The 22nd and 26th carbons of the ergostane skeleton in withaferin A and related steroidal compounds are oxidized to form a six-membered delta lactone unit. NMR spectral analysis identifies C3 in the unsaturated A ring as the main nucleophilic target site for ethyl mercaptan, thiophenol and L-cysteine ethyl ester in vitro.[3] A library of 2, 3-dihydro-3β-substituted derivatives are synthesized by regio/stereoselective Michael addition to ring A.
Regulation
Transcription factor NF-κB in vitro
NF-κB is a transcription factor that regulates many genes involved in cell survival, growth, immune response and angiogenesis. Withaferin A inhibits NF-κB at a very low concentration by targeting the ubiquitin-mediated proteasome pathway (UPP) in endothelial cells.[2] In vitro experiments demonstrated that withaferin A inhibits other transcription factors including Ap1[4] and Sp1.[5]
Biosynthesis
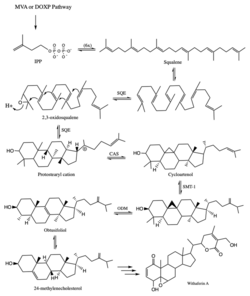
In the Withania somnifera plant, the withaferin A is present in the leaves. Withanolides are terpenoids, which are synthesized in plants using isoprenoids as precursors. Isoprenoids can be synthesized through mevalonate or 1-deoxy-D-xylulose 5-phosphate pathways. Isoprenogenesis significantly governs withanolide synthesis.[6]
Isoprenoids form squalene, which then goes through a variety of intermediate steps to form 24-methylenecholesterol - the sterol precursor of the withanolides.[7]
The biosynthesis of withaferin A uses enzymes such as squalene epoxidase (SQE), cycloartenol synthase (CAS), sterol methyl transferase (SMT), and obtusifoliol-14 –demethylase (ODM).[8]
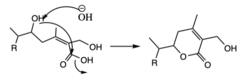
To produce withaferin A from 24-methylene cholesterol, the molecule undergoes several functional changes including formation of a ketone, epoxide, 2 hydroxyl groups, and lactone ring.[9]
See also
References
- ↑ Kupchan, S. M.; Anderson, W. K.; Bollinger, P.; Doskotch, R. W.; Smith, R. M.; Renauld, J. A.; Schnoes, H. K.; Burlingame, A. L. et al. (1969-12-01). "Tumor inhibitors. XXXIX. Active principles of Acnistus arborescens. Isolation and structural and spectral studies of withaferin A and withacnistin". The Journal of Organic Chemistry 34 (12): 3858–3866. doi:10.1021/jo01264a027. PMID 5357526.
- ↑ 2.0 2.1 Mohan, R; Hammers, HJ; Bargagna-Mohan, P; Zhan, XH; Herbstritt, CJ; Ruiz, A; Zhang, L; Hanson, AD et al. (2004). "Withaferin A is a potent inhibitor of angiogenesis". Angiogenesis 7 (2): 115–122. doi:10.1007/s10456-004-1026-3. PMID 15516832.
- ↑ 3.0 3.1 Vanden Berghe, Wim; Sabbe, Linde; Kaileh, Mary; Haegeman, Guy; Heyninck, Karen (2012-11-15). "Molecular insight in the multifunctional activities of Withaferin A". Biochemical Pharmacology 84 (10): 1282–1291. doi:10.1016/j.bcp.2012.08.027. PMID 22981382. https://biblio.ugent.be/publication/3032265/file/3033118.
- ↑ Braun, Lesley; Cohen, Marc (2015-03-30) (in en). Herbs and Natural Supplements, Volume 2: An Evidence-Based Guide. Elsevier Health Sciences. ISBN 978-0-7295-8173-8. https://books.google.com/books?id=Y951BwAAQBAJ.
- ↑ Prasanna Kumar, S; Shilpa, P; Salimath Bharati, P (2009). "Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor". Current Trends in Biotechnology and Pharmacy 3 (2): 138–148. ISSN 0973-8916. http://www.indianjournals.com/ijor.aspx?target=ijor:ctbp&volume=3&issue=2&article=003.
- ↑ Chaurasiya, Narayan D.; Sangwan, Neelam S.; Sabir, Farzana; Misra, Laxminarain; Sangwan, Rajender S. (2012). "Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal)". Plant Cell Reports 31 (10): 1889–1897. doi:10.1007/s00299-012-1302-4. PMID 22733207.
- ↑ Lockley, William J.S.; Rees, Huw H.; Goodwin, Trevor W. (1976). "Biosynthesis of steroidal withanolides in Withania somnifera". Phytochemistry 15 (6): 937–939. doi:10.1016/S0031-9422(00)84374-5. Bibcode: 1976PChem..15..937L.
- ↑ Pandey, Shiv S.; Singh, Sucheta; Pandey, Harshita; Srivastava, Madhumita; Ray, Tania; Soni, Sumit; Pandey, Alok; Shanker, Karuna et al. (2018). "Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis". Scientific Reports 8 (1): 5450. doi:10.1038/s41598-018-23716-5. PMID 29615668. Bibcode: 2018NatSR...8.5450P.
- ↑ Bharitkar, Yogesh P.; Kanhar, Satish; Suneel, Neradibilli; Mondal, Susanta Kumar; Hazra, Abhijit; Mondal, Nirup B. (2015). "Chemistry of withaferin-A: Chemo, regio, and stereoselective synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A via one-pot three-component [3+2] azomethine ylide cycloaddition and their cytotoxicity evaluation". Molecular Diversity 19 (2): 251–261. doi:10.1007/s11030-015-9574-6. PMID 25749788.
 |
