Chemistry:Wulff–Dötz reaction
This article relies too much on references to primary sources. (February 2023) (Learn how and when to remove this template message) |
| Wulff–Dötz reaction | |
|---|---|
| Named after | William Wulff Karl Heinz Dötz |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000681 |
The Wulff–Dötz reaction (also known as the Dötz reaction or the benzannulation reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne and carbon monoxide to give a Cr(CO)3-coordinated substituted phenol.[1][2][3] Several reviews have been published.[4][5] It is named after the German chemist Karl Heinz Dötz (b. 1943) and the American chemist William D. Wulff (b. 1949) at Michigan State University.[6] The reaction was first discovered by Karl Dötz and was extensively developed by his group and W. Wulff's group. They subsequently share the name of the reaction.
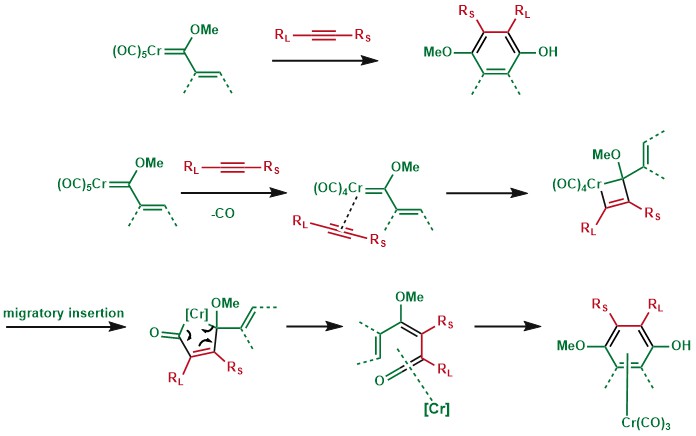
The position of the substituents is highly predictable with the largest alkyne substituent (RL) neighboring the phenol and the smallest alkyne substituent (RS) neighboring the methoxy group.[7][8] Hence, this reaction is more useful for terminal alkynes than internal alkynes.
The phenol can be liberated from the chromium complex by a mild oxidation, such as ceric ammonium nitrate or air oxidation.
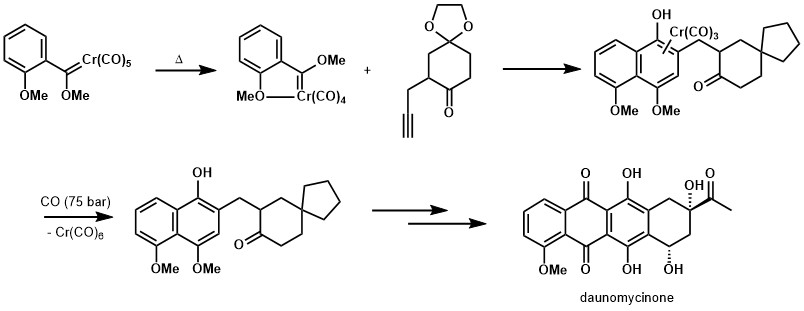
Since this reaction can quickly generate complex phenolic compounds, the Wulff–Dötz reaction has been used most often in the synthesis of natural products, especially Vitamins E and K.[9][10] It is also applicable to the synthesis of polyphenolic compounds, such as calixarenes.[11]
Mechanism
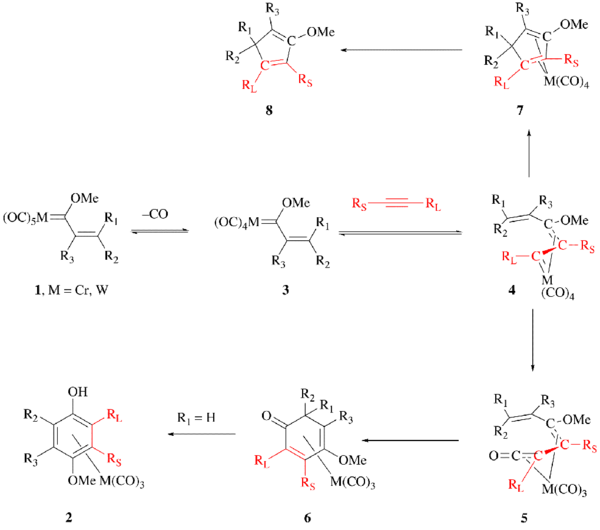
The mechanism is thought to begin with the loss of carbon monoxide from the Fischer carbene complex 1 to give intermediate 3. The loss of CO is rate limiting making the investigation of this reaction mechanism difficult, since all subsequent steps occur rapidly. The alkyne then coordinates to the metal center, a low-energy barrier process. The resulting alkyne complex rearranges to intermediate 4.[12] The η1, η3-complex shown as 4 subsequently undergoes CO insertion to give the η4-vinylketene complex 5, which undergoes electrocyclization to give intermediate 6. When R1 is hydrogen, intermediate 6 is short lived and proceeds to the metal tricarbonyl arene complex 2. Without CO insertion, the reaction proceeds through 7 to the cyclopentadiene product 8.
Examples
Exposing Fischer carbene with alkenyl side chain to an alkyne gives a highly substituted phenol. The phenolic carbon is originated from the CO ligand. The α,β-unsaturated part could also be from an electron rich aryl system, yielding a polycyclic aromatic system. This reaction was first discovered by Karl Dötz and was extensively developed by his group, thus giving the name Dötz reaction. It is sometimes called Wuff-Dötz reaction because William Wuff's group at Michigan State University also extensively contributed to the development of this reaction.[13]
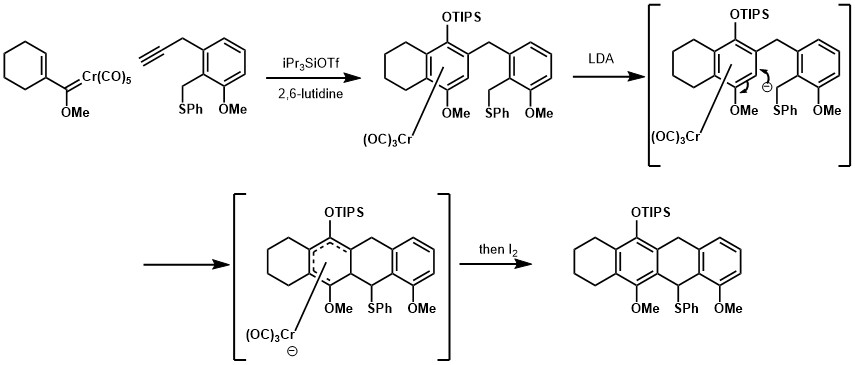
The half-sandwich complex in the Dötz reaction can be demetallated to give corresponding aryl product, or it could be further employed for a nucleophilic addition to aromatic system strategy for synthesis of fully-substituted benzene ring.[14]
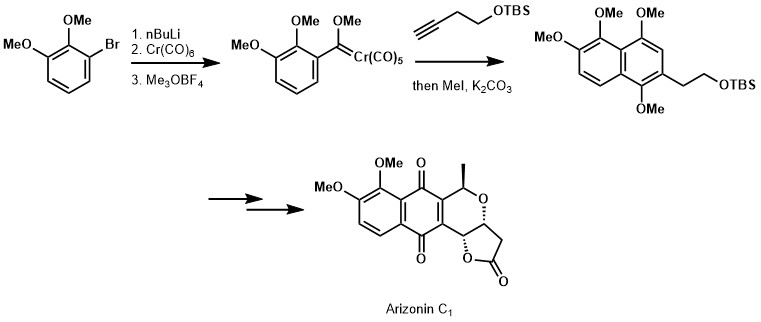
The Dötz reaction has been employed in thee syntheses of natural products, as illustrated below.[15] [16]
Interrupted Dötz reaction
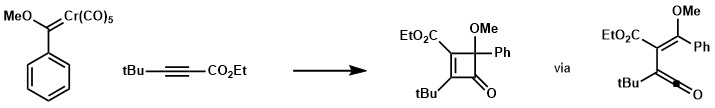
In several cases, if the reactivity of the reagent does not meet or the conditions for Dotz mechanism to operate are not fulfilled, products derived from the interrupted Dotz reaction could be dominant. For instance, if the substituents on alkyne are too bulky, cyclobutene product would be observed instead.[17]
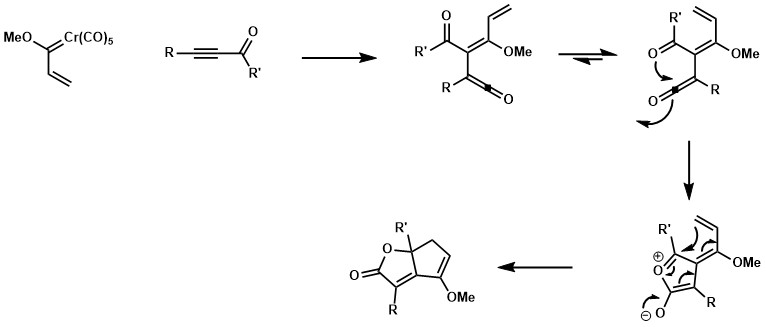
If the alkyne partner bearing a ketone substituent and both R and R’ are not bulky enough, a favored conformation for an 8e pi cyclization could be dominant leading to a fused bicyclic lactone system.[18] [19] [20]
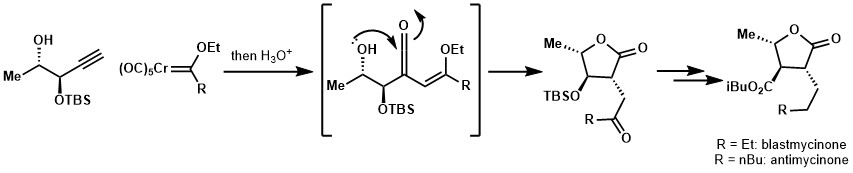
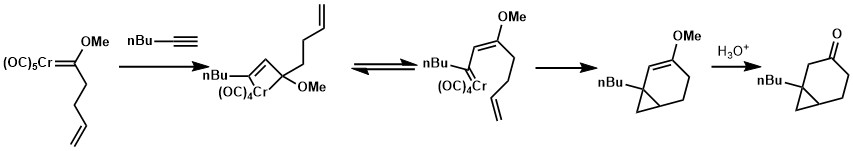
Alkene or nucleophilic moiety on the side chain of alkyne partner could trap the resulting ketene through a [2+2] cycloaddition or nucleophilic addition respectively. This strategy was applied for the syntheses of blastmycinone and antimycinone.[21][22]
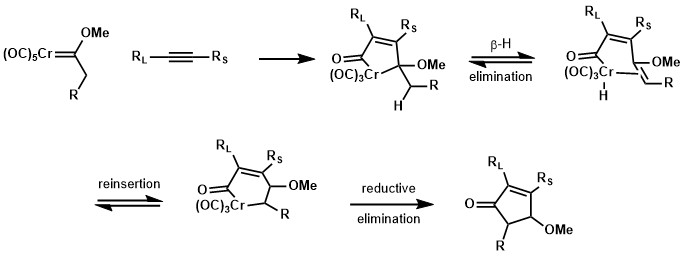
Fischer carbenes with an α-hydrogen could form could give cyclopentenone product similar to Pauson-Khand reaction. This is presumably because of a β-hydride elimination and reinsertion process.[23]
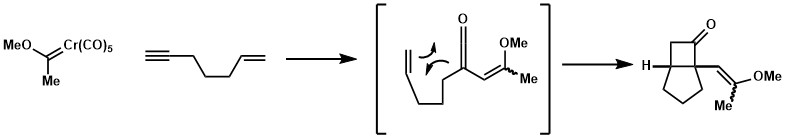
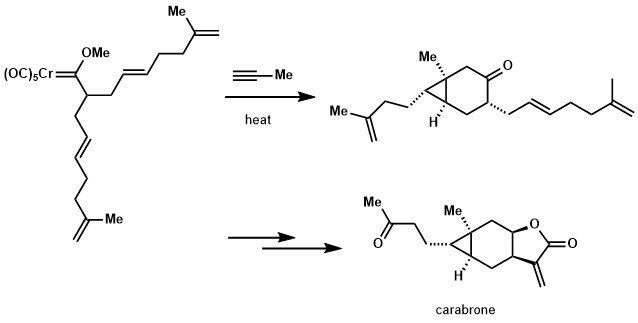
If the alkene moiety is present in Fischer carbene, but not in conjugation, cyclopropanation could be observed. The strategy was employed in a formal synthesis of carabrone.[24][25]
References
- ↑ Dötz, Karl Heinz (September 1975). "Synthesis of the Naphthol Skeleton from Pentacarbonyl-[methoxy(phenyl)carbene]chromium (O) and Tolan". Angewandte Chemie International Edition in English 14 (9): 644–645. doi:10.1002/anie.197506442.
- ↑ Heinz Dötz, Karl; Dietz, Robert; von Imhof, Alexander; Lorenz, Hans; Huttner, Gottfried (June 1976). "Reaktionen von Komplexliganden, IV. Stereoselektive Synthese substituierter Naphthaline: Darstellung und Struktur eines Tricarbonyl(naphthalin)chrom(0)-Komplexes". Chemische Berichte 109 (6): 2033–2038. doi:10.1002/cber.19761090610.
- ↑ Timko, M.; Yamashita, Ayako (1993). "Synthesis of 2-Substituted Naphthalenediol Derivatives Using Chromium Carbene Complexes: 1-Acetoxy-2-Butyl-4-Methoxynaphthalene". Org. Synth. 71: 72. doi:10.15227/orgsyn.071.0072.
- ↑ Waters, Marcey; William, Wulff (2008). "The Synthesis of Phenols and Quinones via Fischer Carbene Complexes". Organic Reactions 70: 121–623. doi:10.1002/0471264180.or070.02. ISBN 978-0471264187.
- ↑ Dotz, K. H. (1 January 1983). "Carbon-carbon bond formation via carbonyl-carbene complexes". Pure and Applied Chemistry 55 (11): 1689–1706. doi:10.1351/pac198355111689.
- ↑ "The Wulff Group at Michigan State University". http://www2.chemistry.msu.edu/faculty/wulff/myweb26/index.htm. Retrieved 23 April 2015.
- ↑ Wulff, William D.; Tang, Peng Cho; McCallum, J. Stuart (December 1981). "Regiochemistry of the reaction of chromium-carbene complexes with acetylenes". Journal of the American Chemical Society 103 (25): 7677–7678. doi:10.1021/ja00415a058.
- ↑ Chamberlin, Steven; Wulff, William D. (June 1994). "Synthons for the Parent Vinyl Carbene Complex in the Benzannulation Reaction". The Journal of Organic Chemistry 59 (11): 3047–3054. doi:10.1021/jo00090a024.
- ↑ Rawat, Manish; Wulff, William D. (February 2004). "Total Synthesis of Carbazoquinocin C: Application of the Benzannulation of Fischer Carbene Complexes to Carbazole-3,4-quinone Alkaloids". Organic Letters 6 (3): 329–332. doi:10.1021/ol0360445. PMID 14748585.
- ↑ White, James D.; Smits, Helmars (January 2005). "Application of the Dötz Reaction to Construction of a Major Portion of the Ansa Macrocycle (−)-Kendomycin". Organic Letters 7 (2): 235–238. doi:10.1021/ol047779s. PMID 15646966.
- ↑ Fernandes, Rodney; Mulay, Sandip (28 May 2014). "Chiral Cups (Calixarenes) via Dötz Benzannulation". Synthesis 46 (14): 1836–1846. doi:10.1055/s-0033-1339122.
- ↑ Hofmann, Peter; Hämmerle, Martin (July 1989). "The Mechanism of the Dötz Reaction: Chromacyclobutenes by Alkyne–Carbene Coupling?". Angewandte Chemie International Edition in English 28 (7): 908–910. doi:10.1002/anie.198909081.
- ↑ Dötz, K. H.; Tomuschat, P. (1999). "Annulation reactions of chromium carbene complexes: scope, selectivity and recent developments". Chemical Society Reviews 28 (3): 187–198. doi:10.1039/A801442F.
- ↑ Chamberlin, Steven; Wulff, William D. (December 1992). "Sequential benzannulation/nucleophilic aromatic addition reactions mediated by chromium(0)". Journal of the American Chemical Society 114 (26): 10667–10669. doi:10.1021/ja00052a090.
- ↑ Wulff, William D.; Tang, Peng Cho (January 1984). "Anthracycline synthesis with Fischer carbene complexes". Journal of the American Chemical Society 106 (2): 434–436. doi:10.1021/ja00314a037.
- ↑ Mahlau, Manuel; Fernandes, Rodney A.; Brückner, Reinhard (2011-06-21). "First Synthesis of the Pyrano-Naphthoquinone Lactone (-)-Arizonin C1". European Journal of Organic Chemistry: n/a. doi:10.1002/ejoc.201100599.
- ↑ Yamashita, A.; Toy, A. (January 1986). "Regioselectivity of the reaction of a chromium-carbene complex with alkynes: Examination of steric and electronic factors". Tetrahedron Letters 27 (30): 3471–3474. doi:10.1016/S0040-4039(00)84825-X.
- ↑ Brandvold, Timothy A.; Wulff, William D.; Rheingold, Arnold L. (February 1990). "Efficient entry to bicyclic lactones via van Halban-White cyclizations". Journal of the American Chemical Society 112 (4): 1645–1647. doi:10.1021/ja00160a063.
- ↑ Brandvold, Timothy A.; Wulff, William D.; Rheingold, Arnold L. (July 1991). "Chromium-mediated cyclizations of cross-conjugated ketoketenes in 8- and 10e- processes". Journal of the American Chemical Society 113 (14): 5459–5461. doi:10.1021/ja00014a051.
- ↑ Waters, Marcey L.; Brandvold, Timothy A.; Isaacs, Lyle; Wulff, William D.; Rheingold, Arnold L. (September 1998). "Stereoelectronic Effects on Product Formation from the E - and Z -Isomers of η 1 ,η 3 -Vinyl Carbene Complexed Intermediates in the Reactions of Fischer Carbene Complexes with Alkynes". Organometallics 17 (19): 4298–4308. doi:10.1021/om980509r.
- ↑ Wulff, William D.; Kaesler, Ralph W. (August 1985). "Cyclobutanone formation via in situ generated vinyl ketene complexes of chromium". Organometallics 4 (8): 1461–1463. doi:10.1021/om00127a028.
- ↑ Ishibashi, Taro; Ochifuji, Nagisa; Mori, Miwako (August 1996). "New lactone synthesis using a chromium carbene complex". Tetrahedron Letters 37 (34): 6165–6168. doi:10.1016/0040-4039(96)01338-X.
- ↑ Challener, Cynthia A.; Wulff, William D.; Anderson, Benjamin A.; Chamberlin, Steve; Faron, Katherine L.; Kim, Oak K.; Murray, Christopher K.; Xu, Yao Chang et al. (February 1993). "Cyclopentenone formation via hydrogen activation in the reactions of chromium carbene complexes with alkynes". Journal of the American Chemical Society 115 (4): 1359–1376. doi:10.1021/ja00057a020.
- ↑ Parlier, A.; Rudler, H.; Platzer, N.; Fontanille, M.; Soum, A. (May 1985). "Interactions des alcynes avec les complexes carbéniques du tungstène portant une double liaison carbone— carbone: Accès aux dérivés bicyclo[4,1,0] heptaniques par insertion-cyclopropanation". Journal of Organometallic Chemistry 287 (1): c8–c12. doi:10.1016/0022-328X(85)80079-6.
- ↑ Hoye, Thomas R.; Vyvyan, James R. (June 1995). "Polycyclic Cyclopropanes from Reactions of Alkene-Containing Fischer Carbene Complexes and Alkynes: A Formal Synthesis of (.+-.)-Carabrone". The Journal of Organic Chemistry 60 (13): 4184–4195. doi:10.1021/jo00118a040.
 |