Chemistry:Xanthydrol
From HandWiki
Short description: Organic compound derived from xanthone reduction
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Xanthen-9-ol | |
| Other names
Xanthanol, 9-Hydroxyxanthene, 9-Xanthydrol, Xanthen-9-ol, 9-Xanthenol, Xanthydrol solution
| |
| Identifiers | |
3D model (JSmol)
|
|
| 10395 | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
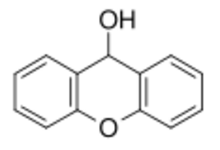
| C13H10O2 | |
| Molar mass | 198.221 g·mol−1 |
| Melting point | 124 to 126 °C (255 to 259 °F; 397 to 399 K)[1] |
| Hazards[2] | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H315, H319, H335, H411 | |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthydrol is an organic chemical compound. Its formula is C13H10O2. Its total molecular weight is 198.221 g/mol. Xanthydrol is used to test the levels of urea in the bloodstream.[3]
Synthesis
Xanthydrol can be produced by the reduction of xanthone.
See also
References
- ↑ Goldberg; Wragg (1957). "972. Spasmolytics derived from xanthen". Journal of the Chemical Society: 4823–4829. doi:10.1039/JR9570004823.
- ↑ "9H-Xanthen-9-ol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/72861#section=Safety-and-Hazards.
- ↑ Bowden, R. S. T. (1962). "The Estimation of Blood Urea by the Xanthydrol Reaction". Journal of Small Animal Practice 3 (4): 217–218. doi:10.1111/j.1748-5827.1962.tb04191.x.
 |

