Chemistry:Yttrium perchlorate
From HandWiki
(Redirected from Chemistry:Yttrium(III) perchlorate)
| |
| Names | |
|---|---|
| Other names
Yttrium triperchlorate, yttrium(III) perchlorate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
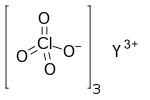
| Y(ClO4)3 | |
| Molar mass | 387.244 |
| Appearance | liquid |
| Density | g/cm–3 |
| soluble | |
| Hazards | |
| Main hazards | Oxidizer |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| P210, P220, P260, P264, P280, P301+330+331, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P321, P363, P370+378, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Yttrium perchlorate is the inorganic compound with the chemical formula Y(ClO4)3.[1][2] The compound is an yttrium salt of perchloric acid.[3][4]
Synthesis
Dissolving yttrium oxide in perchloric acid solution can produce yttrium perchlorate octahydrate.[citation needed]
Chemical properties
Potentially explosive.[5]
Physical properties
The compound is soluble in water and forms a hexahydrate with the formula Y(ClO4)3•6H2O.[6][7]
References
- ↑ "Yttrium(III) Perchlorate Solution". American Elements. https://www.americanelements.com/yttrium-iii-perchlorate-solution-14017-56-2.
- ↑ "CAS 14017-56-2 Yttrium perchlorate - Alfa Chemistry". alfa-chemistry.com. https://www.alfa-chemistry.com/yttrium-perchlorate-cas-14017-56-2-item-64771.htm.
- ↑ "Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade, Thermo Scientific". Fisher Scientific. https://www.fishersci.be/shop/products/yttrium-iii-perchlorate-50-w-w-aq-soln-reagent-grade-thermo-scientific/11460311.
- ↑ Macintyre, Jane E. (13 November 1994) (in en). Dictionary of Inorganic Compounds, Supplement 2. CRC Press. p. 585. ISBN 978-0-412-49100-9. https://books.google.com/books?id=yV-Kj-ubc0IC&dq=Yttrium(III)+perchlorate&pg=PA585. Retrieved 15 March 2023.
- ↑ Macintyre, Jane E. (23 July 1992) (in en). Dictionary of Inorganic Compounds. CRC Press. p. 2931. ISBN 978-0-412-30120-9. https://books.google.com/books?id=9eJvoNCSCRMC&dq=Yttrium(III)+perchlorate&pg=PA2931. Retrieved 15 March 2023.
- ↑ "Yttrium Perchlorate, Hydrated, 50% Solution, Reagent". gfschemicals.com. https://www.gfschemicals.com/Product/yttrium-perchlorate-hydrated-50-percent-solution-reagent-cas-14017-56-2-item-532-sku-77402.
- ↑ "40580 Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade". Alfa Aesar. https://www.alfa.com/ru/catalog/040580/.
 |

