Chemistry:Zincke nitration
From HandWiki
| Zincke nitration | |
|---|---|
| Named after | Theodor Zincke |
| Reaction type | Substitution reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000413 |
The Zincke nitration is a nitration reaction in which a bromine is replaced by a nitro group on an electron-rich aryl compound such as a phenol or cresol. Typical reagents are nitrous acid or sodium nitrite. The reaction is a manifestation of nucleophilic aromatic substitution and is named after Theodor Zincke, who first reported it in 1900.[1][2]
Two examples:[3]
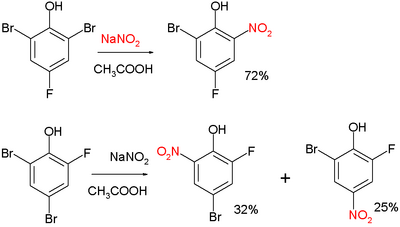
and:[4]
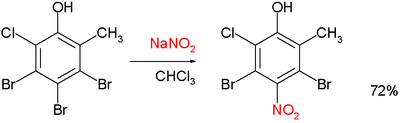
The Zincke nitration should not be confused with the Zincke–Suhl reaction or the Zincke reaction.
See also
References
- ↑ Zincke, Th. (1900). "Ueber die Einwirkung von salpetriger Säure auf Brom- und Chlorderivate von Phenolen" (in German). J. Prakt. Chem. 61 (1): 561–567. doi:10.1002/prac.19000610145. http://gallica.bnf.fr/ark:/12148/bpt6k90826q/f571.image.r=journal%20praktische%20chemie.langDE.
- ↑ Zincke, Th. (1900). "Ueber die Einwirkung von Salpetersäure auf Halogenderivate des p-Kresols" (in German). J. Prakt. Chem. 63 (1): 183–187. doi:10.1002/prac.19010630111. http://gallica.bnf.fr/ark:/12148/bpt6k90828d/f192.image.r=Journal%20f%C3%BCr%20praktische%20Chemie.langDE.
- ↑ Raiford, L. Chas.; LeRosen, Arthur L. (1944). "The Nitration of Brominated Fluorophenols by the Zincke Method". J. Am. Chem. Soc. 66 (11): 1872–1873. doi:10.1021/ja01239a020.
- ↑ Raiford, L. Chas.; Miller, Glen R. (1933). "Behavior of Mixed Halogenated Phenols in the Zincke Method of Nitration". J. Am. Chem. Soc. 55 (5): 2125–2131. doi:10.1021/ja01332a059.
 |

